Part:BBa_K2560008
Phytobrick version of BBa_B0034
This is the Phytobrick version of the ribosome binding site BBa_B0034 and was build as a part of the Marburg Collection. Instructions of how to use the Marburg Collection are provided at the bottom of the page.
Overview
The ribosome binding site (RBS) is a short sequence upstream the transcription start. It can be identified easily by the typical Shine-Dalgarno sequence that pairs with the anti Shine-Dalgarno sequence at the 3’ end of the 16S rRNA ( Smit MH and Duin J van., 1993). The effectiveness of this sequence is determined by its base-pairing potential to the ribosome and its spacing from the start codon ( Smit MH and Duin J van., 1994). The presence of the RBS is crucial for protein translation.
Characterization
For quantifying the RBS strengths, unfortunately, we could not use our favorite reporter the lux operon because in case of this operon, each CDS possesses its own RBS. Therefore, replacing the RBS upstream of the first CDS (LuxA) alone does not suffice to achieve a difference in reporter expression dependent on the RBS strength. Consequently we used sfGFP, the reporter that showed the second best performance in our initial reporter experiment (Link to Measurement).
We built test constructs that are identical in all used parts except for the used RBS. The resulting data are shown in figure 7. The sample labeled with “empty” represents V. natriegens with a plasmid without a reporter. Apparently, the difference between the tested constructs expressing sfGFP in different amounts does not differ much from the non expressing control. However, it is possible to obtain some information about the order of the tested RBS. The strongest signal was observed for B0034 followed by B0030. These RBS are also described as strong in the iGEM registry. In case of B0031 and B0032, the measured signal is lower than for the non-sfGFP expressing strain. Therefore no conclusion can be provided for these two parts.
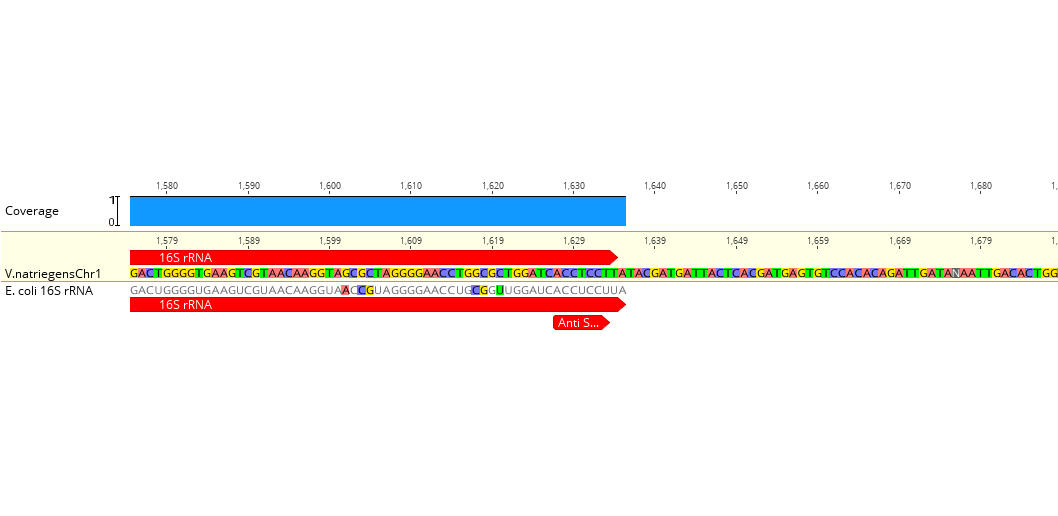
We expected that the order of the strength of the tested RBS should be similar to E. coli. Prior to the experiment, we created an alignment of the 16S rRNA of both organisms and found that the Anti-Shine-Dalgarno sequence, the bases that are responsible for binding the Shine-Dalgarno sequence on the mRNA, do not differ between both organisms (figure 8).
On the measurement page (Link to Measurement). we suggested the lux operon as the reporter of choice for all experiments that focus on measuring transcriptional activity . We see the RBS characterization as the confirmation that using sfGFP as reporter does not yield reliable data for very weak expression.
In future experiments, enzymatic reporters such as LacZ or β-glucuronidase (GUS) could be tested for their suitability in experiments for the quantification of translational efficiency or post translational effects (e.g. degradation tags)
Marburg Toolbox
We proudly present the Marburg Collection, a novel golden-gate-based toolbox containing various parts that are compatible with the PhytoBrick system and MoClo. Compared to other bacterial toolboxes, the Marburg Collection shines with superior flexibility. We overcame the rigid paradigm of plasmid construction - thinking in fixed backbone and insert categories - by achieving complete de novo assembly of plasmids.
36 connectors facilitate flexible cloning of multigene constructs and even allow for the inversion of individual transcription units. Additionally, our connectors function as insulators to avoid undesired crosstalk.
The Marburg Collection contains 123 parts in total, including:
inducible promoters, reporters, fluorescence and epitope tags, oris, resistance cassettes and genome engineering tools. To increase the value of the Marburg Collection, we additionally provide detailed experimental characterization for V. natriegens and a supportive software. We aspire availability of our toolbox for future iGEM teams to empower accelerated progression in their ambitious projects.
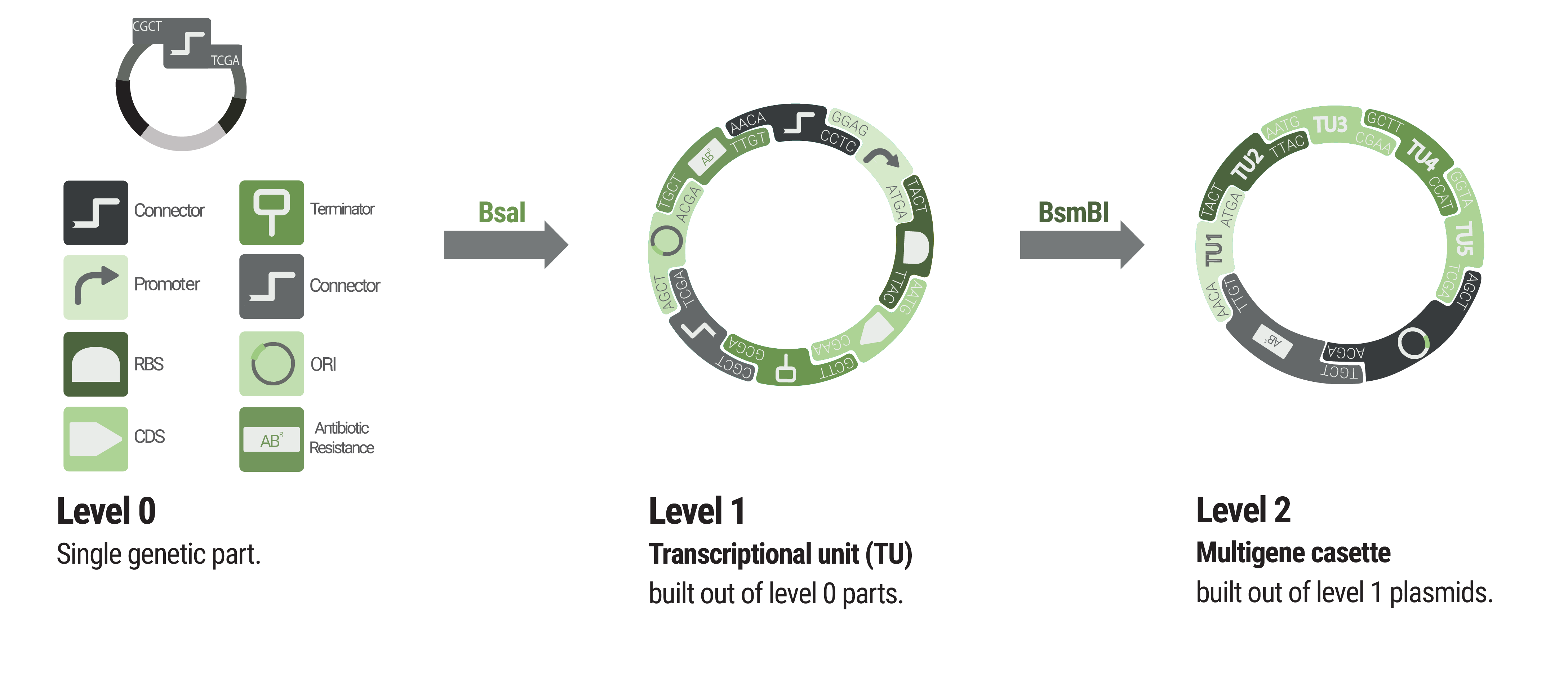
Basic building blocks like promoters or terminators are stored in level 0 plasmids. Parts from each category of our collection can be chosen to built level 1 plasmids harboring a single transcription unit. Up to five transcription units can be assembled into a level 2 plasmid.
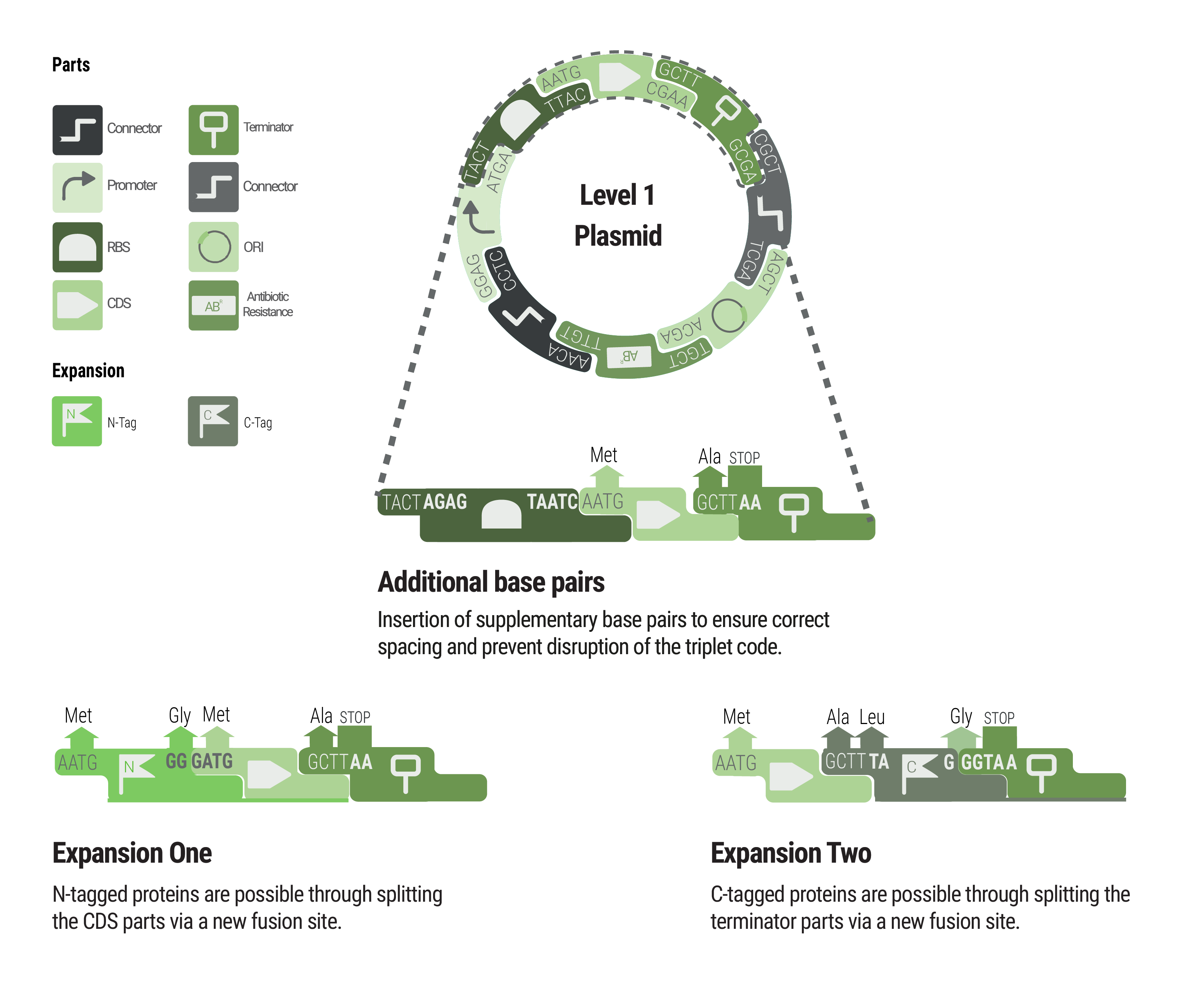
Between some parts, additional base pairs were integrated to ensure correct spacing and to maintain the triplet code. We expanded our toolbox by providing N- and C- terminal tags by creating novel fusions and splitting the CDS and terminator part, respectively.
Parts of the Marburg Toolbox

- K2560011 (5'Connector Dummy)
- K2560055
(1-6
Connector) - K2560065 (5'Con1)
- K2560066 (5'Con2)
- K2560067 (5'Con3)
- K2560068 (5'Con4)
- K2560069 (5'Con5)
- K2560075 (5'Con1
Short Res) - K2560076 (5'Con2
Short) - K2560077 (5'Con3
Short) - K2560078 (5'Con4
Short) - K2560079 (5'Con5
Short) - K2560095 (5'Con1 inv)
- K2560096 (5'Con2 inv)
- K2560097 (5'Con3 inv)
- K2560098 (5'Con4 inv)
- K2560099 (5'Con5 inv)
- K2560105 (5'Con5 inv
Ori) - K2560107 (5'Con1
Res)

- K2560007 (J23100)
- K2560009 (J23104)
- K2560014 (J23106)
- K2560015 (J23115)
- K2560017 (J23101)
- K2560018 (J23102)
- K2560019 (J23103)
- K2560020 (J23105)
- K2560021 (J23107)
- K2560022 (J23108)
- K2560023 (J23109)
- K2560024 (J23110)
- K2560025 (J23111)
- K2560026 (J23113)
- K2560027 (J23114)
- K2560028 (J23116)
- K2560029 (J23117)
- K2560030 (J23118)
- K2560031 (J23119)
- K2560123
(pTet) - K2560124 (pTrc)
- K2560131 (Promoter Dummy)

- K2560012 (3'Connector Dummy)
- K2560070 (3'Con1)
- K2560071 (3'Con2)
- K2560072 (3'Con3)
- K2560073 (3'Con4)
- K2560080 (3'Con5 Ori)
- K2560100 (3'Con1 inv
Short) - K2560101 (3'Con2 inv
Short) - K2560102 (3'Con3 inv
Short) - K2560103 (3'Con4 inv
Short) - K2560104 (3'Con5 inv
Short) - K2560106 (3'Con1 inv
Short Res) - K2560108 (3'Con1 inv)
- K2560109 (3'Con1 inv
Res) - K2560110 (3'Con2 inv)
- K2560111 (3'Con3 inv)
- K2560112 (3'Con4 inv)
- K2560113 (3'Con5 inv)

- K2560048 (Cam. Res. RFP)
- K2560056
(Kan. Res. (pSB3K3) RFP) - K2560057
(Kan. Res. (pSB3K3) GFP) - K2560058
(Tet. Res. (pSB3T5) RFP) - K2560059
(Tet. Res. (pSB3T5) GFP) - K2560125 (Carb. Res. RFP)
- K2560126 (Carb. Res. GFP)
- K2560127 (Carb. Res. into BBa_K2560002)
- K2560132 (Cam. Res. into BBa_K2560002)
- K2560133
(Kan. Res. into BBa_K2560002) - K2560134
(Tet. Res. into BBa_K2560002)
Tags and Entry Vectors
| None |