Part:BBa_K3757004
c_CHEN_6
Cyclotide MCoTI-II with Trypsin inhibitor activity from Momordica cochinchinensis. The antimicrobial peptide CHEN is grafted into loop 6 of the cyclotide, a His6-tag is grafted into loop 5. This part can be cloned into Oak1 precursor and co-expressed with CtAEP1 to yield as a cyclic peptide in Nicotiana benthamiana.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Contents
Usage and Biology
MCoTI-II is a squash trypsin inhibitor cyclotide, also known as cyclic knottins, and originates from the plant species Momordica cochinchinensis1. Cyclotides are a class of plant cyclic peptides with a length of around 30 amino acids (aa). They comprise three disulfide bridges, which together with their cyclic backbone form the characteristic cyclic cystine knot motif making the cyclotide structure very rigid. This motif is responsible for cyclotides’ exceptional stability towards proteases and heat. The regions between their six cysteine residues are called loops 1 to 6. Cyclotides are classified into three categories by their sequence: Möbius, bracelet and trypsin inhibitor cyclotides with the latter having a low sequence conservation compared to the other two classes.2 In plants, these peptides are expressed as precursors, which are finally localized to the vacuole. There, the actual cyclotide sequences are recognized by specialized cyclizing asparaginyl endopeptidases (AEPs), which are catalyzing the backbone cyclization.3 MCoTI-II itself is 34 aa in length, with the sequence GGVCPKILKKCRRDSDCPGACICRGNGYCGSGSD, and is folded into a cystine stabilized triple-stranded beta-sheet. Its natural function as a trypsin inhibitor is based on its inhibitory loop 1. Thereby, it acts as a defensive agent against the plant’s predators.1 MCoTI-II does not disrupt cell membranes and does not display antibacterial, nor hemolytic activity.4
Cyclotides like MCoTI-II have been used for grafting approaches in the past. Thereby, grafting means the insertion into or the replacement of one of the cyclotide’s loops with another peptide sequence (figure 1). This enables combining the cyclotide’s stability with the inserted peptide’s biological activity4. Regarding MCoTI-II, successful grafting into loops 1, 3, 5 and 6 has been demonstrated5.
This biological part is a grafted construct of MCoTI-II designed and tested by iGEM team Tuebingen 2021. Two different antimicrobial peptides (AMPs) were used and either grafted into loop 1 or loop 6 of the cyclotide. In addition, a His6-tag (BBa_M50428) was grafted into loop 5 for affinity purification and immunodetection. Furthermore, flexible GS-linkers were included at the interface of the cyclotide’s native sequence and the grafted peptide. For further information on the project, please visit project description wiki page of team Tuebingen 2021. The different grafted constructs used by iGEM Tuebingen are listed in table 1.
| Table 1: The grafted cyclotide constructs iGEM team Tuebingen 2021 worked with in the wetlab; the vectors in which the constructs were cloned are shown as well. | ||||
|---|---|---|---|---|
| BBa_K3757002 | c_blank | MCoTI-II loop 5 His6 | X | |
| BBa_K3757003 | c_CHEN_1 | MCoTI-II loop 1 CHEN, loop 5 His6 | X | X |
| BBa_K3757004 | c_CHEN_6 | MCoTI-II loop 6 CHEN, loop 5 His6 | X | X |
| BBa_K3757005 | c_KR-12_1 | MCoTI-II loop 1 KR-12, loop 5 His6 | X | |
| BBa_K3757006 | c_KR-12_6 | MCoTI-II loop 6 KR-12, loop 5 His6 | X | |
This construct c_CHEN_6 is MCoTI-II with the AMP CHEN grafted into loop 6, as well as a His6-tag grafted into loop 5. Its sequence and peptide segments are displayed in figure 2.
CHEN is a modified peptide fragment of the AMP chensinin-1b, which itself is a strongly modified analogue of the naturally occurring 18 aa long AMP chensinin-1. Chensinin-1 occurs on the skin of the frog species Rana chensinensis and is active against gram-positive bacteria. The 18 aa long analogue chensinin-1b is active against both gram-positive and gram-negative bacteria. Its NMR structure displays a central alpha-helical region and an N-terminal random-coil region. To optimize the antimicrobial properties of chensinin-1b, the truncated and modified peptide [R4,R10]C1b(3–13) – hereafter called CHEN – was created. CHEN is 11 aa long and has the sequence VWRRWRRFWRR. The alternating hydrophobic (V, W) and cationic (R) residues lead to an amphipathic character in its alpha-helical fold, with a hydrophobic and a cationic face of the helix. This fold was shown to be essential for the antibacterial activity of CHEN, which involves the fast disruption of cell membranes. CHEN shows minimal inhibitory concentrations (MIC) of 1.56 μM to 3.13 μM against different clinically relevant gram-positive and gram-negative bacterial species, making it a potent antibacterial agent. Furthermore, it does not exhibit hemolytic activity up to a concentration of 500 μM.6
The grafted construct was cloned into an Oak1 precursor peptide (BBa_K3757007) and expressed in N. benthamiana, regulated by a CaMV 35S promoter (BBa_K788000) and a 35S terminator (BBa_K1159307), and C-terminally tagged with a c-myc-tag (BBa_K3757008). This is summarized as the composite part BBa_K3757007. This composite part was cloned into a vector with the genes encoding CtAEP1 (BBa_K3757001) and GFP (BBa_K3669012) as a reporter gene, forming a 3in1 vector (BBa_K3757011). Also, as a control, a 2in1 vector was constructed, which misses the CtAEP1 encoding gene. The resulting vector can then be used for transient transfection of N. benthamiana leaves to express the stabilized AMPs. For further information, visit experiments wiki page of team Tuebingen 2021.
Modeling
Building the Structures
To predict the final structures with which we would run our simulations later on, we used AlphaFold8. After predicting the structures, we wrote a script that utilizes the Python package Modeller10 to cyclize the structures, meaning it joins their termini. The final cyclized structures were compared to the wild-type version of MCoTI-II. We calculated the root-mean-square-deviation (RMSD), a measure as to how far the atoms of two structures are spaced apart at average, given in Ångström, using PyMOL9 and its “align” command. For wild-type KR-12 we used a structure from PDB and for wild-type CHEN we predicted the structure using I-TASSER.
The RMSD of our final structure of c_CHEN_6 compared to MCoTI-II is 1.297 Å.
Molecular Dynamics Simulations
Molecular Dynamics simulation is the process of computing the interactions of different atoms/molecules over a certain timeframe and then visualizing/analyzing the resulting data. The interactions between different atoms are computed using force fields, which are databases with definitions of how strong and with what kind of force certain atoms interact with each other. It is possible to compute all-atom systems, where all atoms are computed as individual entities, which we did.
- Our first assumption was that the AMPs interact with the bacterial membrane, as they were characterized to do so by the literature. They are effective against gram-positive and gram-negative bacteria.6
- The second assumption our model includes is the use of a model gram-negative bacterial membrane containing a 3:1 ratio of zwitterionic to anionic phospholipids. The zwitterionic phospholipid is POPE (palmitoyloleoylphosphatidylethanolamine) and the anionic one is POPG (palmitoyloleoylphosphatidylglycerol).11.
We used the Molecular Dynamics simulation suite GROMACS12,13, which is used for preparing and running simulations.
All our all-atom systems were built by our partner team IISER Kolkata as part of our partnership. After we provided them with the structures of our constructs built above, they used them to build the membrane systems and prepared the scripts for running those. After some slight modifications to make the simulations run-ready, we ran them using our supercomputer access.
To analyze our simulations we used “Visual Molecular Dynamics”14 (VMD in short), Grace, as well as GROMACS itself.
The following parameters were analyzed regarding our all-atom simulations:
- Root Mean Square Deviation (RMSD): as we expected the membrane to be more stable than the protein, we expected the protein to be stabilized once binding occurs (blue line in figure 3)
- Solvent Accessible Surface (SASA): we expected the SASA to decrease once the protein binds to the membrane, as part of its surface gets blocked (orange line in figure 3)
- Hydrogen bonds (H-Bonds): we expected the number of hydrogen bonds to increase once the protein and membrane come into contact (black line in figure 3)
To make it easier to compare the fluctuation of the parameters at different time points, figure 4 shows the running averages (of 50 datapoints) of the mentioned parameters within one graph for the AMP CHEN.
As the values are quite close to 0 and therefore correlation is low, it can be said that no other parameter is as representative as the number of hydrogen bonds generated.
In case of RMSD this might be due to the fact that its values were already low even when the protein hadn’t bound to the membrane.
Furthermore, the membrane itself is flexible as well, not holding the protein in one place as initially thought.
For SASA, a possible explanation could be that the protein wasn’t close enough to the molecules of the membrane to effectively reduce the amount of surface reachable by water molecules.
To compare the constructs to each other, we computed the arithmetic mean of the number of hydrogen bonds created over the simulation:
| Table 2: Average number of hydrogen bonds over the course of the simulation. | |
|---|---|
| Construct | Average number of hydrogen bonds |
| c_KR-12_1_CHEN_6 | 5.87306347 |
| KR-12 | 5.0225 |
| c_blank | 3.626 |
| CHEN | 5.82 |
In our simulations, c_KR-12_1_CHEN_6 performed best, closely followed by CHEN and KR-12.
Molecular Docking
Molecular Docking is the process of finding the optimal docking position of a ligand to a receptor (usually an enzyme) in a 3-dimensional space. It is sometimes used to screen a library of ligands against each other to find the best-fitting ligand for an enzyme. The score of a certain docking position of a ligand is called the binding affinity and is calculated using an energy function in kcal/mol. The position with the lowest binding energy is the one with the highest binding affinity and therefore the optimal calculated position.
To build our model, we assumed the following things:
- Our AMPs, namely KR-12 and CHEN, are both positively charged. Therefore, they should bind to the membrane by leveraging the opposing charge, meaning using anionic phospholipids.
- The anionic phospholipid POPG, also known as palmitoyloleoylphosphatidylglycerol, is an anionic phospholipid that is abundant in bacterial membranes11.
To run our molecular docking, we needed the structures of our ligands (AMPs, grafted constructs) and receptor (POPG). We used the same ligand structures as in all our simulations and got a POPG membrane structure from the GROMACS download site15 from which we extracted a single POPG molecule.
We used PyRx16 to prepare the ligands and the receptor by automatically letting the program add hydrogens, choose polar hydrogens, add charges, and do necessary further preprocessing. This didn’t work for one structure and so we prepared it using AutoDockTools17,18.
To calculate the docking of our constructs, we used AutoDock Vina19, a tool for running Molecular Docking. We ran the simulations on the supercomputer BinAC.
The calculated binding affinity for CHEN is -3.2 kcal/mol but c_CHEN_6 produced no sensible docking. AutoDock Vina produces multiple modes of how the ligand could be docked to the receptor (we chose to produce 5 per ligand). We tried to choose the ones with the highest binding affinities for our model, while also filtering out impossible configurations which occurred because it seems that Vina cannot dock circular molecules and therefore breaks the bond between the termini (which wouldn’t happen in a natural system).
The binding mode of CHEN can be found in figure 4. CHEN side chains are colored pink, KR-12 side chains are colored cyan, POPG is colored orange and the peptide itself is colored gray with dark-blue side chains. The visualizations and analysis of the bonds were created using the Protein-Ligand Interaction Profiler20.
File:T--Tuebingen--chen complex.mp4
Expression in Nicotiana benthamiana
Green fluorescence from infiltrated N. benthamiana leaves could be observed for both the 2in1 (figure 5) and the 3in1 vector (figure 6), which shows successful infiltration and transient expression of genes from these vectors.
Extraction and Purification
SDS-PAGE and Western Blots
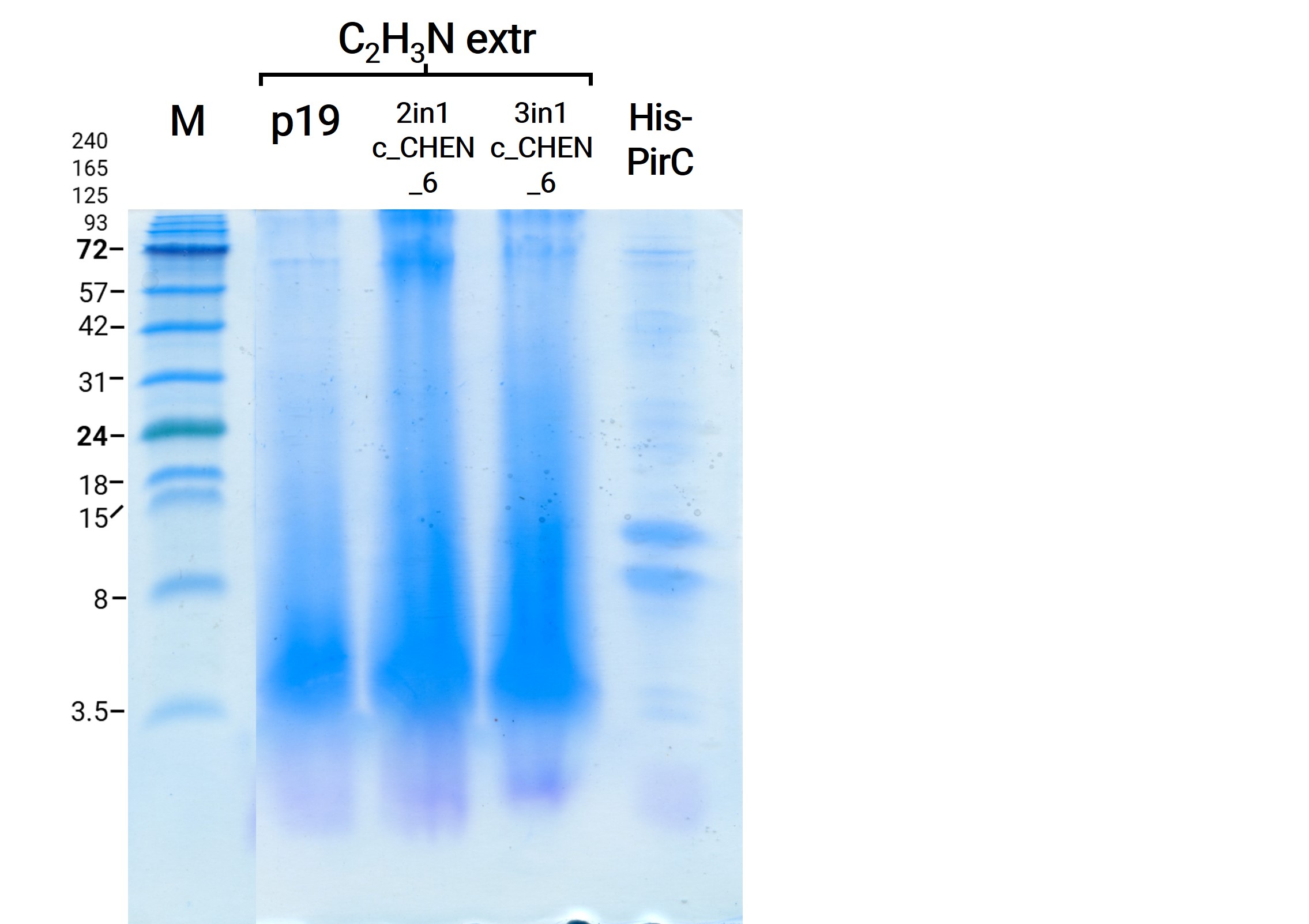
We resuspend our in liquid nitrogen crushed plant extracts in Lämmli buffer. After a short incubation on ice, the samples were denaturated by heating to 95 °C, followed by direct application on an SDS-PAGE. All samples showed clearly more distinct bands of all kDa sizes on the Coomassie-stained SDS-gel in comparison to the prior gels, as visible in figure 7. For example, the bands in all lanes at the size of about 53 kDa are most likely from the large subunit of the enzyme Rubisco (Ribulose bisphosphate carboxylase), a plant enzyme involved in photosynthesis.

In addition, we performed a western blot of the very same samples with an anti-His-tag primary antibody to be able to specifically detect our His-tagged cyclotides. An immunoblot signal at a size of about 18 kDa could be detected (figure 8), which raised the suspicion that cyclization of our cyclotides by the AEP did not work, as the precursor of our constructs is about the same size as the detected signal. Another possible explanation is that the band shows indeed our cyclotide, which does not run at the expected molecular range in the SDS-PAGE. This could be due to its highly positive charge, which might not be completely masked by the negatively charged SDS or due to its cyclic structure. Unfortunately, no signal was detected for our positive control, a His-tagged protein of about 84 kDa. This could be explained by the huge size difference between this control and our peptide. Thus, it could have happened that the blotting of the control on the membrane did not work.
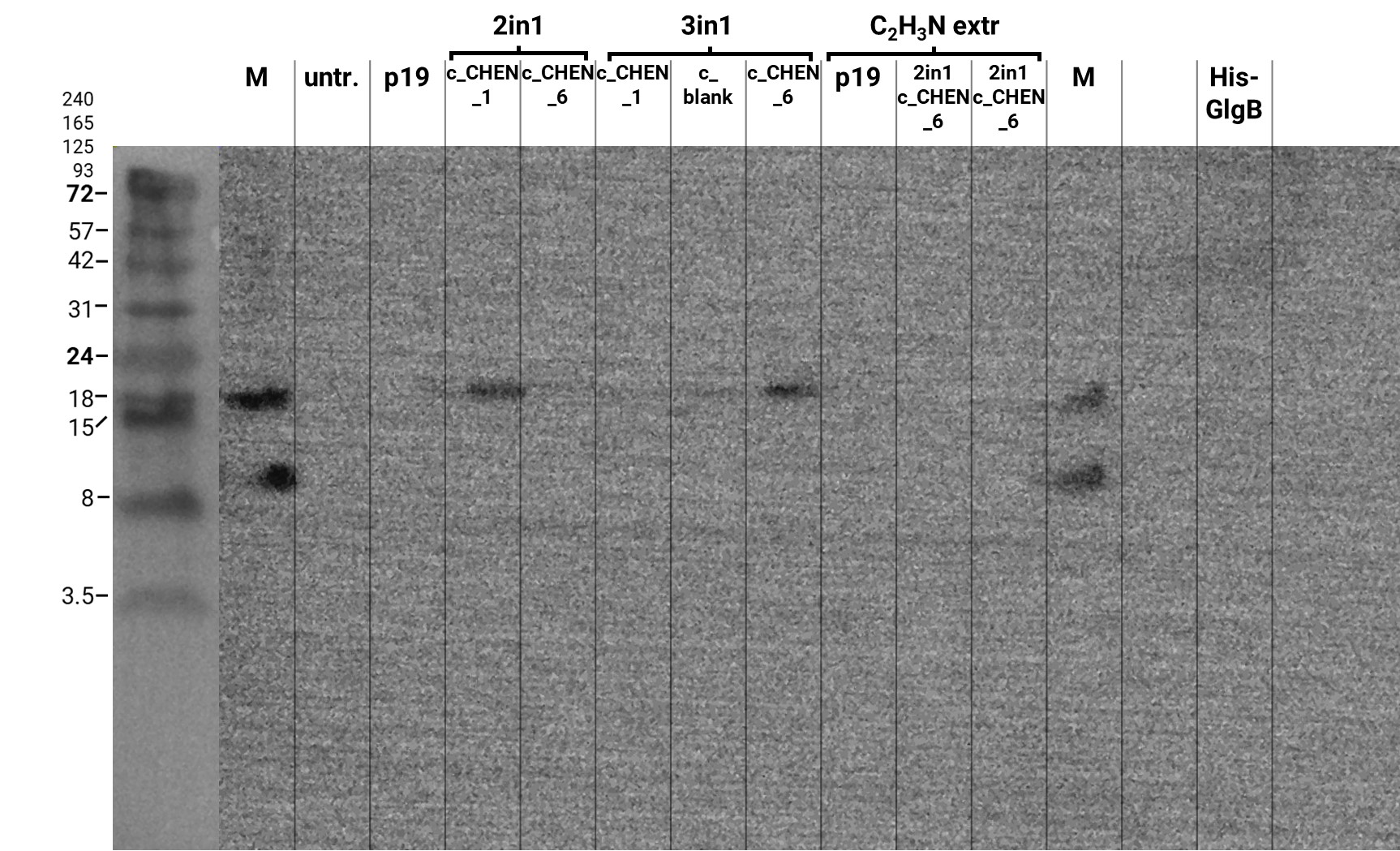
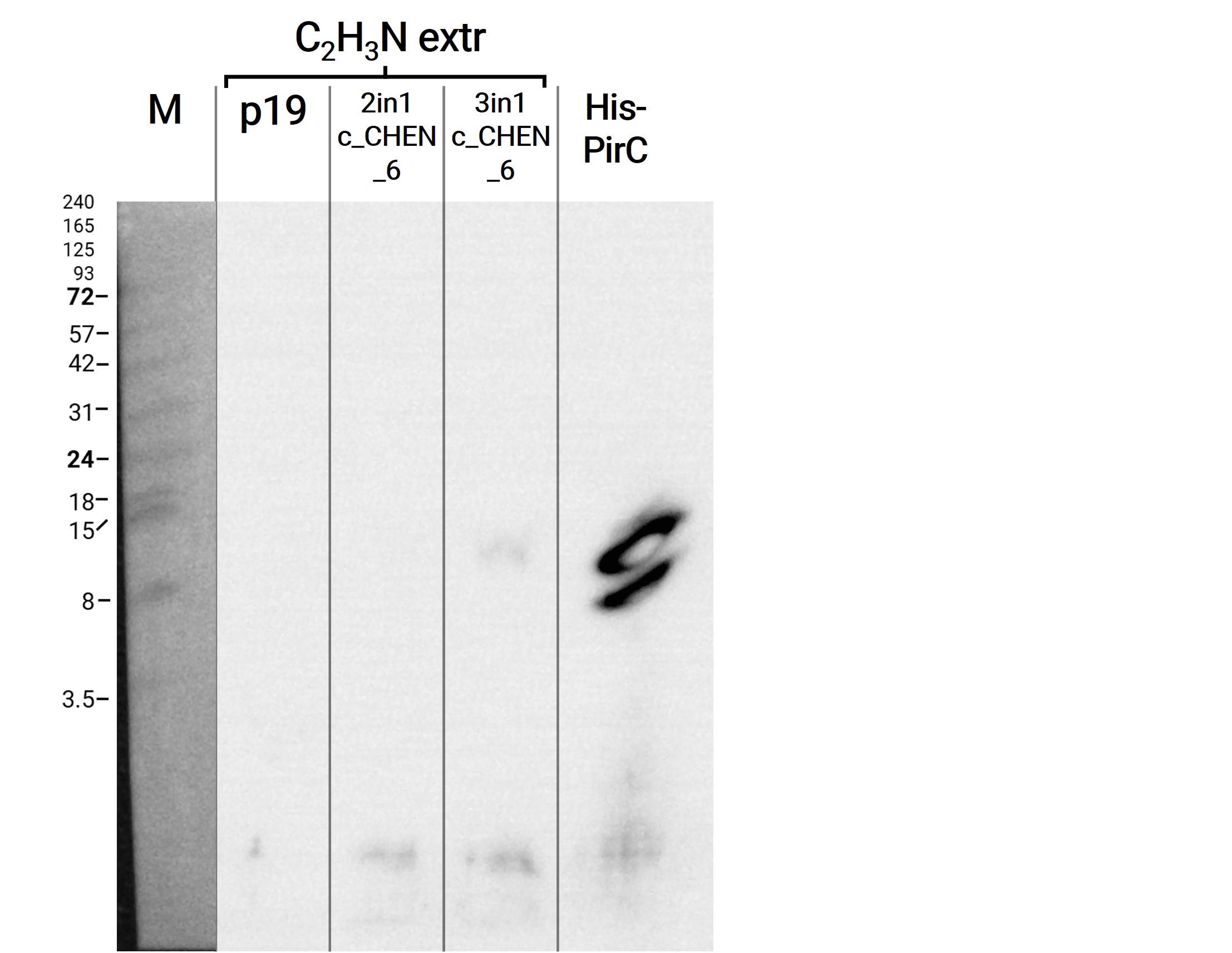
The results from the leaves, which were extracted using an acidic-acetonitrile based buffer are also displayed on the gel and blot (Figure 7 and 8). For this method (adapted from Poon et al, 2018)26 we used dried leaf material and evaporated the extraction solvent in the last step. Prior to applying these samples onto the SDS-PAGE some of the extract was therefore solubilized in ddH2O and then mixed with 2X Lämmli buffer in equal ratio. As can be seen in figure 2, the lanes of these samples show an irregular picture, as no clear and distinct bands can be identified apart from one apparently quite big band at the height of 3.5 kDa. For these samples no signal could be detected in the western blot (figure 9).
Because of the unusual bands in the gel, we decided to repeat this experiment and applied freshly prepared samples onto a Tricine-SDS gel as well as performed a western blot afterwards. The gel looks very similar to the first attempt (compare figure 7 and 9) but on the blot we were able to detect some signal this time (figure 10). However, the molecular weight (MW) range (smaller than 3.5 kDa) in which the signal occurs does not correspond to anything we expect. Only in the lane of the construct 3in1 c_CHEN_6 a weak band can be observed at the MW of about 8 kDa, which would be around the expected size of our cyclotide construct. But as the signal is very low, this assumption would have to be verified by further experiments.
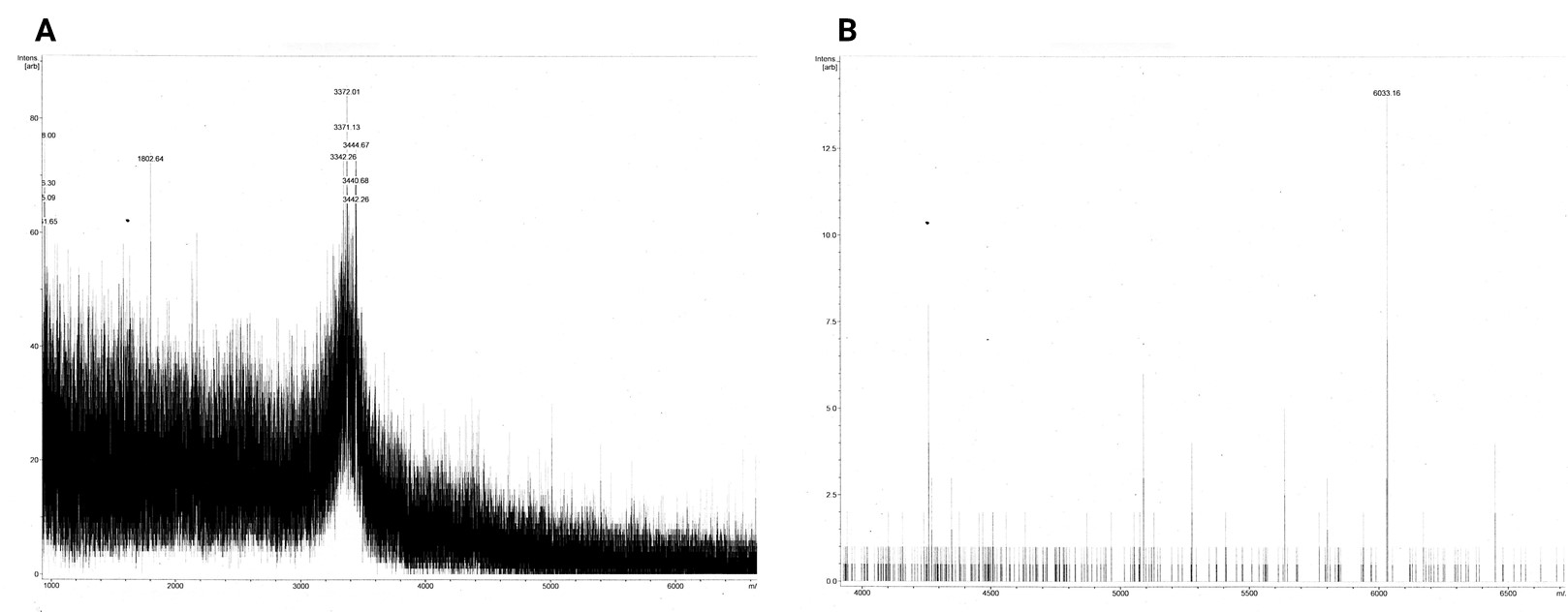
MALDI-TOF MS
The dissolved acetonitrile extracts were additionally concentrated and desalted using C18 resin. The MALDI-TOF MS spectrum of 2in1 construct c_CHEN_6 showed no fitting peaks. However, many peaks corresponding to m/z ratios around 3400 were visible (figure 11 A). These peaks fit single-charge M+ ions of peptides making up the broad band visible at around 3.5 kDa in the Coomassie-stained SDS-PAGE gel of the acetonitrile extracts. Therefore, we concluded that our cyclotides are unlikely to be represented by this 3.5 kDa band. The spectrum of 3in1 construct c_CHEN_6 showed a low-intensity peak with a m/z ratio of 6033.16 (figure 11 B). This value is similar to the average mass of a single-charged M+ ion of cyclic c_CHEN_6, which is expected to be 6041.89 Da with all cys residues reduced, and 6035.89 Da with all cys residues oxidized, as would be expected since no reducing agent was used on this construct.
Antimicrobial assays
Inhibition Zone Assays
As an easy and fast method to estimate the antimicrobial activity of our antimicrobial peptides (AMPs) we used inhibition zone assays. Generally, agar plates are inoculated with a known concentration of test microorganism and then filter paper discs containing the compound to be tested are placed on the agar. The germination and growth of bacteria is inhibited as the antimicrobial substance diffuses into the agar and as a read out the diameters of inhibition growth zones are measured.21
Therefore, Escherichia coli (E. coli) BW25113 and Bacillus subtilis (B. subtilis) 168 were grown as overnight cultures in LB medium and those were used to inoculate new cultures. When they reached middle logarithmic growth phase (OD600 = 0.8 - 1.0) they were diluted either 1:10 or 1:50 and spread onto LB-agar plates. Small filter paper discs were placed onto the agar plates and 20 µl of sample were pipetted onto each disc. The plates were incubated over night at 37 °C.
We used the crude extract of the transfected Nicotiana benthamiana leaves as well as the purified peptides for testing. As positive controls we applied the chemically synthesized AMPs CHEN and KR-12 as these are grafted into our cyclotide construct. We also tested leaves from plants that were transfected with the construct of the empty cyclotide scaffold (c_blank) as a negative control. All samples were solubilized in HEPES buffer (100 mM HEPES 150 mM NaCl, 3 mM KCl, pH 7.5), thus an influence of the buffer system can be ruled out.
After incubation of the agar plates O.N. we observed inhibition zones around the filter plates, on which our positive controls were applied for both bacterial strains, which is shown in figure 12 and figure 13, respectively. However, we used much higher concentrations (100 µM and 10 mM) than the minimal inhibitory concentration (MIC) that can be found in the literature for the respective AMPs (MIC(CHEN) = 3.13 µM, MIC(KR-12) = 2.5 µM)22,23.
Neither the crude extracts of our infiltrated N. benthamiana leaves nor the purified peptides to be tested showed any inhibition of bacterial growth. The negative control showed no inhibition as well.
The fact that even our positive controls only worked quite high concentrated indicates that the inhibition zone assay is unsuitable for the determination of the antimicrobial activity of AMPs. This might be due to interactions with the LB medium, which might shield the activity of the negatively charged AMPs as this charge is crucial for their functionality. This has been observed by other researchers before24. Therefore, we decided not to use these assays anymore for the testing of our peptides but focused on microdilution assays.


Microdilution Assays
Two-fold microdilution assays were performed in order to obtain quantitative results. In each well of a 96-well plate, bacterial suspension of E. coli or B. subtilis overnight cultures were aliquoted. Samples were added in a two-fold diluting manner and plates were incubated at 37°C for 24h. Before (t0) and after (t1) incubation, OD600 was measured. We calculated bacterial growth with the following equation: t1 - t0. Since all data are represented as delta t, the influence of intrinsic colors of extracts can be excluded. For cultivation we used brain heart infusion (BHI) and the standard medium for microdilution assays, Mueller-Hinton Broth (MH). However, antimicrobial activity might be hidden by media components24. Therefore, we diluted MH 1 to 5 (MH 1/5) in order to reduce the influence of media on our constructs, as already shown in a recent publication25.
Since we used our crude extracts, we could not determine the concentration of the constructs. Thus, all results are stated as dilution factors of the respective crude extracts. For each sample we performed a serial dilution series starting with the undiluted extract (=1) until a 1/32 (=0,03) dilution. As growth controls we cultivated bacteria in the respective media without any extracts. We used synthesized peptides KR-12 und CHEN diluted in HEPES buffer (100 mM HEPES 150 mM NaCl, 3 mM KCl, pH 7.5) in defined concentrations for positive controls. All conditions were prepared in triplicates and results are stated as mean of each triplicate. Statistical analysis was performed in Origin 2019 using 1-way ANOVA with p-value at 0.05.

As already assumed, different media showed different effects of our constructs. This is due to the fact that some media conditions can hide the antimicrobial activity24,25. Since we did not observe any activity in BHI, neither for our constructs nor for the control peptides, we excluded these results in the following discussion. In the control peptides, we did not observe growth inhibition of E. coli in MH medium. In contrast, B. subtilis is sensitive to the control peptides and bacterial cell growth is significantly reduced already with a 31,3 µM concentration (figure 14). With the control peptides we observed in MH media diluted to 1/5 (MH 1/5) an inhibition in bacterial growth in both, E. coli and B. subtilis (figure 15) However, the growth control of both bacteria strains in MH 1/5 showed lower cell growth compared to undiluted MH media. Therefore, it might be possible that bacteria are already stressed because of a lack in nutrition and this might make the cell more susceptible to the peptides. Interestingly, in all control samples, except for E. coli in MH 1/5, CHEN showed stronger antimicrobial activity compared to KR-12.

We didn’t observe any effect of our grafted construct crude extracts on bacterial growth in any of the tested media (data not shown).
References
1Heitz, A., Hernandez, J. F., Gagnon, J., Hong, T. T., Pham, T. T., Nguyen, T. M., Le-Nguyen, D., & Chiche, L. (2001). Solution structure of the squash trypsin inhibitor MCoTI-II. A new family for cyclic knottins. Biochemistry, 40(27), 7973–7983. https://doi.org/10.1021/bi0106639
2Craik, D. J., & Conibear, A. C. (2011). The chemistry of cyclotides. The Journal of Organic Chemistry, 76(12), 4805–4817. https://doi.org/10.1021/jo200520v
3Conlan, B. F., Gillon, A. D., Barbeta, B. L., & Anderson, M. A. (2011). Subcellular targeting and biosynthesis of cyclotides in plant cells. American Journal of Botany, 98(12), 2018–2026. https://doi.org/10.3732/ajb.1100382
4Koehbach, J., Gani, J., Hilpert, K., & Craik, D. J. (2021). Comparison of a Short Linear Antimicrobial Peptide with Its Disulfide-Cyclized and Cyclotide-Grafted Variants against Clinically Relevant Pathogens. Microorganisms, 9(6), 1249. https://doi.org/10.3390/microorganisms9061249
5Craik, D. J., & Du, J. (2017). Cyclotides as drug design scaffolds. Current Opinion in Chemical Biology, 38, 8–16. https://doi.org/10.1016/j.cbpa.2017.01.018
6Dong, W., Dong, Z., Mao, X., Sun, Y., Li, F., & Shang, D. (2016). Structure-activity analysis and biological studies of chensinin-1b analogues. Acta Biomaterialia, 37, 59–68. https://doi.org/10.1016/j.actbio.2016.04.003
7Yang, Jianyi; Zhang, Yang (2015): I-TASSER server: new development for protein structure and function predictions. In: Nucleic acids research 43 (W1), W174-81. DOI: 10.1093/nar/gkv342
8Jumper, John; Evans, Richard; Pritzel, Alexander; Green, Tim; Figurnov, Michael; Ronneberger, Olaf et al. (2021): Highly accurate protein structure prediction with AlphaFold. In: Nature. DOI: 10.1038/s41586-021-03819-2
9The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC.
10 Sali, A.; Blundell, T. L. (1993): Comparative protein modelling by satisfaction of spatial restraints. In: Journal of Molecular Biology 234 (3), S. 779–815. DOI: 10.1006/jmbi.1993.1626
11 Lee, Juho; Jung, Sang Won; Cho, Art E. (2016): Molecular Insights into the Adsorption Mechanism of Human β-Defensin-3 on Bacterial Membranes. In: Langmuir 32 (7), S. 1782–1790. DOI: 10.1021/acs.langmuir.5b04113
12Abraham, Mark James; Murtola, Teemu; Schulz, Roland; Páll, Szilárd; Smith, Jeremy C.; Hess, Berk; Lindahl, Erik (2015): GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. In: SoftwareX 1-2, S. 19–25. DOI: 10.1016/j.softx.2015.06.001
13Páll, Szilárd; Abraham, Mark James; Kutzner, Carsten; Hess, Berk; Lindahl, Erik (2015): Tackling Exascale Software Challenges in Molecular Dynamics Simulations with GROMACS. In: Stefano Markidis und Erwin Laure (Hg.): Solving Software Challenges for Exascale, Bd. 8759. Cham: Springer International Publishing (Lecture Notes in Computer Science), S. 3–27.
14Schulten, W. H. (1996). VMD - Visual Molecular Dynamics. Journal of Molecular Graphics, 33-38
15Kukol, Andreas (2009): Lipid Models for United-Atom Molecular Dynamics Simulations of Proteins. In: Journal of chemical theory and computation 5 (3), S. 615–626. DOI: 10.1021/ct8003468
16Dallakyan, Sargis; Olson, Arthur J. (2015): Small-molecule library screening by docking with PyRx. In: Methods in molecular biology (Clifton, N.J.) 1263, S. 243–250. DOI: 10.1007/978-1-4939-2269-7_19.
17Morris, Garrett M.; Huey, Ruth; Lindstrom, William; Sanner, Michel F.; Belew, Richard K.; Goodsell, David S.; Olson, Arthur J. (2009): AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. In: Journal of computational chemistry 30 (16), S. 2785–2791. DOI: 10.1002/jcc.21256.
18 Sanner, M. F. (1999). Python: a programming language for software integration and development. J Mol Graph Model, 17(1), 57-61.
19Trott, O., & Olson, A. J. (2010). AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of computational chemistry, 31(2), 455-461.
20Adasme, Melissa F.; Linnemann, Katja L.; Bolz, Sarah Naomi; Kaiser, Florian; Salentin, Sebastian; Haupt, V. Joachim; Schroeder, Michael (2021): PLIP 2021: expanding the scope of the protein-ligand interaction profiler to DNA and RNA. In: Nucleic acids research 49 (W1), W530-W534. DOI: 10.1093/nar/gkab294.
21Balouiri, M., Sadiki, M., & Ibnsouda, S. K. (2016). Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis, 6(2), 71–79. https://doi.org/10.1016/j.jpha.2015.11.005
22Dong, W., Dong, Z., Mao, X., Sun, Y., Li, F., & Shang, D. (2016). Structure-activity analysis and biological studies of chensinin-1b analogues. Acta Biomaterialia, 37, 59–68. https://doi.org/10.1016/j.actbio.2016.04.003
23Gunasekera, S., Muhammad, T., Strömstedt, A. A., Rosengren, K. J., & Göransson, U. (2020). Backbone Cyclization and Dimerization of LL-37-Derived Peptides Enhance Antimicrobial Activity and Proteolytic Stability. Frontiers in Microbiology, 11, 168. https://doi.org/10.3389/fmicb.2020.00168
24Wiegand, Irith; Hilpert, Kai; Hancock, Robert E. W. (2008): Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. In Nature protocols 3 (2), pp. 163–175. DOI: 10.1038/nprot.2007.521.
25Koehbach, Johannes; Gani, Jurnorain; Hilpert, Kai; Craik, David J. (2021): Comparison of a Short Linear Antimicrobial Peptide with Its Disulfide-Cyclized and Cyclotide-Grafted Variants against Clinically Relevant Pathogens. In Microorganisms 9 (6), p. 1249. DOI: 10.3390/microorganisms9061249.
26Poon, S., Harris, K. S., Jackson, M. A., McCorkelle, O. C., Gilding, E. K., Durek, T., van der Weerden, N. L., Craik, D. J., & Anderson, M. A. (2018). Co-expression of a cyclizing asparaginyl endopeptidase enables efficient production of cyclic peptides in planta. Journal of Experimental Botany, 69(3), 633–641. https://doi.org/10.1093/jxb/erx422
| None |





