Part:BBa_K3757007
35S Oak1
Gene encoding the cyclotide precursor Oak1 (BBa_K3757001), C-terminally tagged with a c-myc tag (BBa_K3757008). Regulated by the eukaryotic constitutive CaMV 35S promoter (BBa_K788000) and the 35S terminator polyadenylation signal (BBa_K1159307). In one Golden Gate cloning step, a sequence encoding a peptide can be cloned into the Oak1 precursor. This gene can then be co-expressed with CtAEP1 in Nicotiana benthamiana leading to expression of the peptide in cyclized form.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 1468
Illegal BamHI site found at 1858 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 66
Contents
Usage and Biology
This part can be used for the constitutive expression of a grafted cyclotide construct embedded in the Oak1 precursor in plants, C-terminally tagged with a c-myc-tag. After expression, the included tag may be used for affinity purification or detection of the precursor with an anti-c-myc-tag antibody in an immunoassay. It inherits the following basic parts:
- CaMV 35S promoter (BBa_K788000)
- Oak1 precursor (BBa_K3757001)
- c-myc-tag (BBa_K3757008)
- 35S terminator (BBa_K1159307)
Oak1 is the precursor protein of the cyclotide kalata B1 from the herb Oldenlandia affinis. Cyclotides are cyclic peptides involved in plant host defense. The native Oak1 precursor harbors the sequence encoding the actual cyclotide, flanked by a N-terminal and a C-terminal propeptide.1 It also contains an ER-signal sequence at its N-terminus, which is cleaved upon localization of the precursor to the ER. In the ER, three disulfide bonds are formed, building the cyclic cystine knot motif, which is characteristic for cyclotides. Afterward, the Oak1 precursor translocates to the vacuole where it is cyclized by the cyclizing asparaginyl endopeptidase (AEP) OaAEP1 and thereby activated. The AEP enzyme recognizes a specific amino acid sequences in the N- and C-terminal propeptides, leading to subsequent cleavage of the propeptides and cyclization of kalata B1.2
iGEM team Tuebingen 2021 used the Oak1 precursor to express stabilized antimicrobial peptides (AMPs) in a cyclotide scaffold. For further information, visit project description wiki page of team Tuebingen 2021. Several adjustments were made to the native Oak1 sequence:
- Team Tuebingen used the AEP CtAEP1 (BBa_K3757001) for cyclization of the cyclotides, which has a very high catalytic efficiency. Compared to OaAEP1, CtAEP1 has different recognition sequence requirements for the cyclotide precursor3. The C-terminal propeptide was therefore removed and replaced by a sequence encoding the dipeptide “HV”.
- The sequence encoding kalata B1 cyclotide was replaced by a lacZ operon. This is composed of lac promoter, lac operator and the lacZ gene encoding β-galactosidase. The operon is flanked by Esp3I type IIS restriction sites allowing scarless replacement of the lacZ operon with an arbitrary DNA sequence in a single Golden Gate cloning step. Introduction of new AMP-encoding sequences into the Oak1 precursor should therefore be easy and efficiently facilitated. Success of this cloning step can be monitored by blue-white selection.
The modified Oak1 precursor was expressed in N. benthamiana, regulated by a CaMV 35S promoter (BBa_K788000) and a 35S terminator (BBa_K1159307), C-terminally tagged with a c-myc-tag (BBa_K3757008). This is summarized as the composite part BBa_K3757007. This composite part was cloned into a vector with the genes encoding CtAEP1 (BBa_K3757001) and GFP (BBa_K3669012) as a reporter gene, forming a 3in1 vector (BBa_K3757011). Also, as a control, a 2in1 vector was constructed, which misses the CtAEP1 encoding gene. In these 3in1 and 2in1 vectors, an arbitrary AMP construct may be inserted in a single cloning step. The resulting vector can then be used for transient transfection of N. benthamiana leaves to express the stabilized AMPs. For further information, please visit the experiments wiki page of team Tuebingen 2021.
Cloning
The correct cloning of the cyclotide with the respective AMP into the Oak1 precursor in our empty 3in1 vector (BBa_K3757011) could also be verified through blue-white screening of the grown colonies on LB-agar plates supplemented with X-Gal and Isopropyl-β-D-thiogalactopyranoside (IPTG) (figure 2). Hence, we could recognize successfully cloned colonies by their color because bacteria carrying the 3in1 plasmid with an AMP construct in their Oak1 precursor appeared white and not blue due to them not expressing β-galactosidase.
Expression in Nicotiana benthamiana
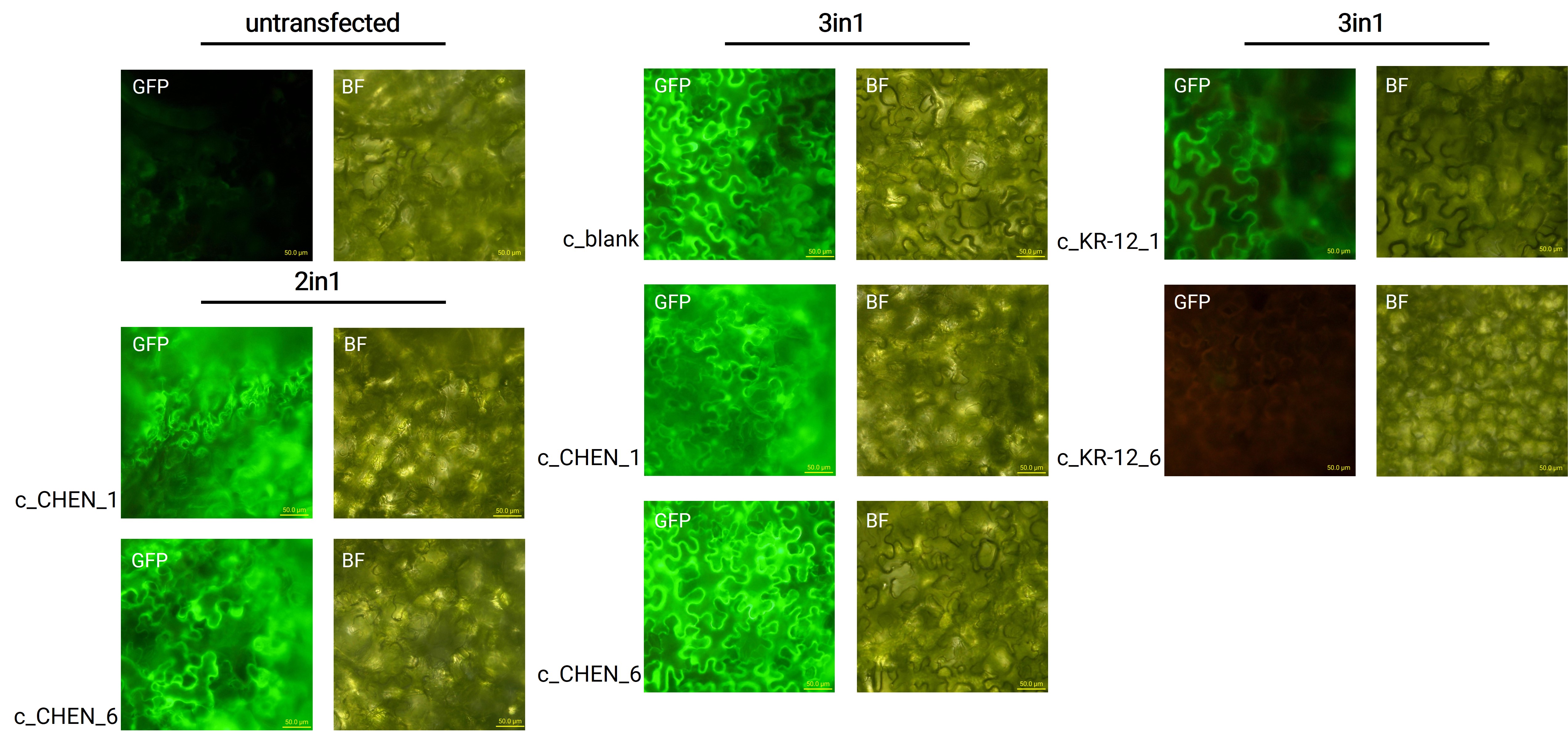
Oak1 precursor containing an AMP encoding sequence was co-expressed with GFP (BBa_K3669012) as a reporter gene. Four days after agroinfiltration of N. benthamiana leaves, leaf pieces were analysed with fluorescence microscopy (figure 3) to confirm GFP expression. In comparison to the negative control, leaves transfected with our final vectors showed clear green fluorescence for all except the c_KR-12_6 construct. As a negative control, non-infiltrated leaves or leaves infiltrated with p19 only were checked for green fluorescence. As shown in figure 3, this negative control showed no significant level of green fluorescence, confirming that the green fluorescence we detected for other vectors can only result from successful expression of one of our final vectors.
We can draw the conclusion that the transfection was successful for both the 2in1 and the 3in1 vectors. However, not the whole infiltrated leaves were fluorescent but defined loci only, which indicates a low expression rate.
Extraction from Nicotiana benthamiana
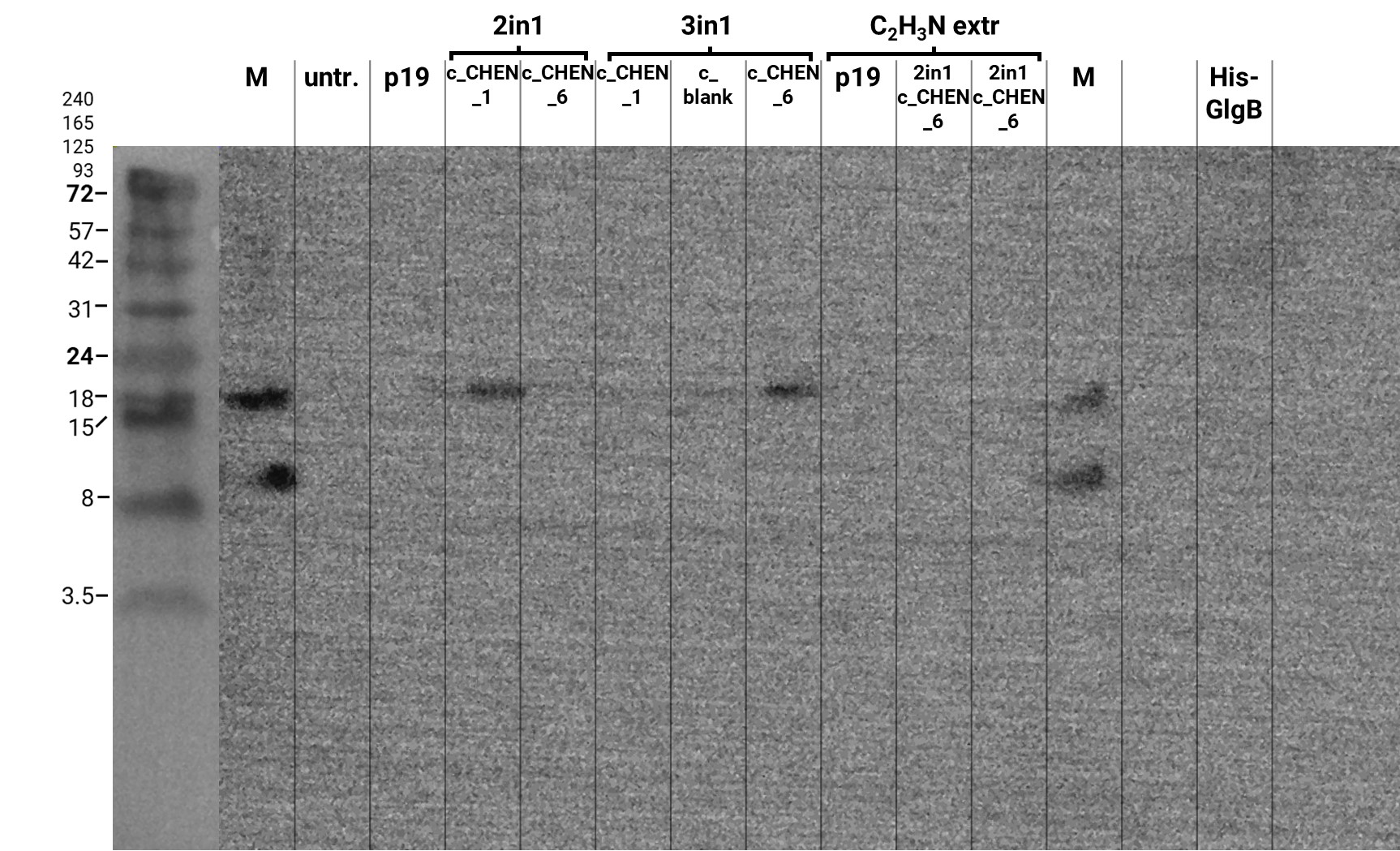
We performed a western blot of the plant crude extract samples, with an anti-His-tag primary antibody to be able to specifically detect our His-tagged cyclotides. An immunoblot signal at a size of about 18 kDa could be detected (figure 4), which raised the suspicion that cyclization of our cyclotides by the AEP did not work, as the Oak1 precursor of our constructs is about the same size as the detected signal. Another possible explanation is that the band shows indeed our cyclotide, which does not run at the expected molecular range in the SDS-PAGE. This could be due to its highly positive charge, which might not be completely masked by the negatively charged SDS or due to its cyclic structure. Unfortunately, no signal was detected for our positive control, a His-tagged protein of about 84 kDa. This could be explained by the huge size difference between this control and our peptide. Thus, it could have happened that the blotting of the control on the membrane did not work.
References
1Poon, S., Harris, K. S., Jackson, M. A., McCorkelle, O. C., Gilding, E. K., Durek, T., van der Weerden, N. L., Craik, D. J., & Anderson, M. A. (2018). Co-expression of a cyclizing asparaginyl endopeptidase enables efficient production of cyclic peptides in planta. Journal of Experimental Botany, 69(3), 633–641. https://doi.org/10.1093/jxb/erx422
2Narayani, M., Babu, R., Chadha, A., & Srivastava, S. (2020). Production of bioactive cyclotides: a comprehensive overview. Phytochemistry Reviews, 19(4), 787–825. https://doi.org/10.1007/s11101-020-09682-9
3 Nguyen, G. K. T [Giang K. T.], Wang, S [Shujing], Qiu, Y., Hemu, X., Lian, Y., & Tam, J. P [James P.] (2014). Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis. Nature Chemical Biology, 10(9), 732–738. https://doi.org/10.1038/nchembio.1586
| None |