Part:BBa_M50428
6x His tag
Tag to detect metal ions
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Contents
Usage by team Tuebingen 2021
We used this part as a tag for affinity purification and immunodetection. In our project, we wanted to express stabilized antimicrobial peptides (AMPs) in Nicotiana benthamiana. Therefore, we grafted selected AMPs into a cyclotide scaffold. Cyclotides are a class peptides originating from plants, which are exceptionally stable due to their cyclic cystine knot structural motif1. Parts of their structure are called loops, and the sequence of another peptide can be inserted into these loops. This is called grafting and allows combining the stability of the cyclotide with the bioactivity of the grafted peptide2. Apart from a selected AMP, we also grafted a His6-tag into our cyclotide constructs. This was done to allow affinity purification of the expressed constructs from plant crude extract, and to allow detection of the peptides with an anti-His-antibody. We used the cyclotide MCoTI-II as scaffold in which the His6-tag was grafted into loop 5. However, there were some difficulties in our approach. Usually, affinity tags are added to either the N- or C-terminus of a protein since this is unlikely to affect its structure or bioactivity. This was not possible for our cyclic peptide, hence it was uncertain whether the His6-tag would disrupt the cyclotide’s structure, whether the tag could be used for affinity purification and whether it would be detectable with an antibody.
Construct design and modeling
We introduced the His6-tag into loop 5 of our cyclotide constructs, flanked by linkers to give it more structural flexibility. As linker sequences, “RG” and “NGY” were used at the tag’s N- and C-terminus, respectively. After designing the sequences, we evaluated them by predicting their structure using AlphaFold3. The predicted structured suggest that the His6-tag does not disturb the peptide fold. Furthermore, the tag seems to be surface exposed and hence it should be accessible. As an example, the predicted structure for our construct c_CHEN_1 (BBa_K3757003) is shown in figure 1. CHEN is one of the AMPs we used for grafting.
Western blot of plant crude extracts
We ordered the designed DNA sequences from a DNA synthesis company. Afterwards, the sequences were cloned into our 2in1 and 3in1 expression constructs (BBa_K3757011), transformed into agrobacteria and used for transient transfection of N. benthamiana. After incubating the plants, the transfected leaves were harvested, homogenized and extracted with different buffers. The crude extracts were separated by SDS-PAGE and used for western blot with an anti-His-tag antibody.
We extracted leaf tissue with Lämmli buffer. In the following western blot, an immunoblot signal at a size of about 18 kDa could be detected (figure 2), which raised the suspicion that cyclization of our cyclotides by the AEP did not work, as the precursor of our constructs is about the same size as the detected signal. Another possible explanation is that the band shows indeed our cyclotide, which does not run at the expected molecular range in the SDS-PAGE. This could be due to its highly positive charge, which might not be completely masked by the negatively charged SDS or due to its cyclic structure. Unfortunately, no signal was detected for our positive control, a His-tagged protein of about 84 kDa. This could be explained by the huge size difference between this control and our peptide. Thus, it could have happened that the blotting of the control on the membrane did not work.
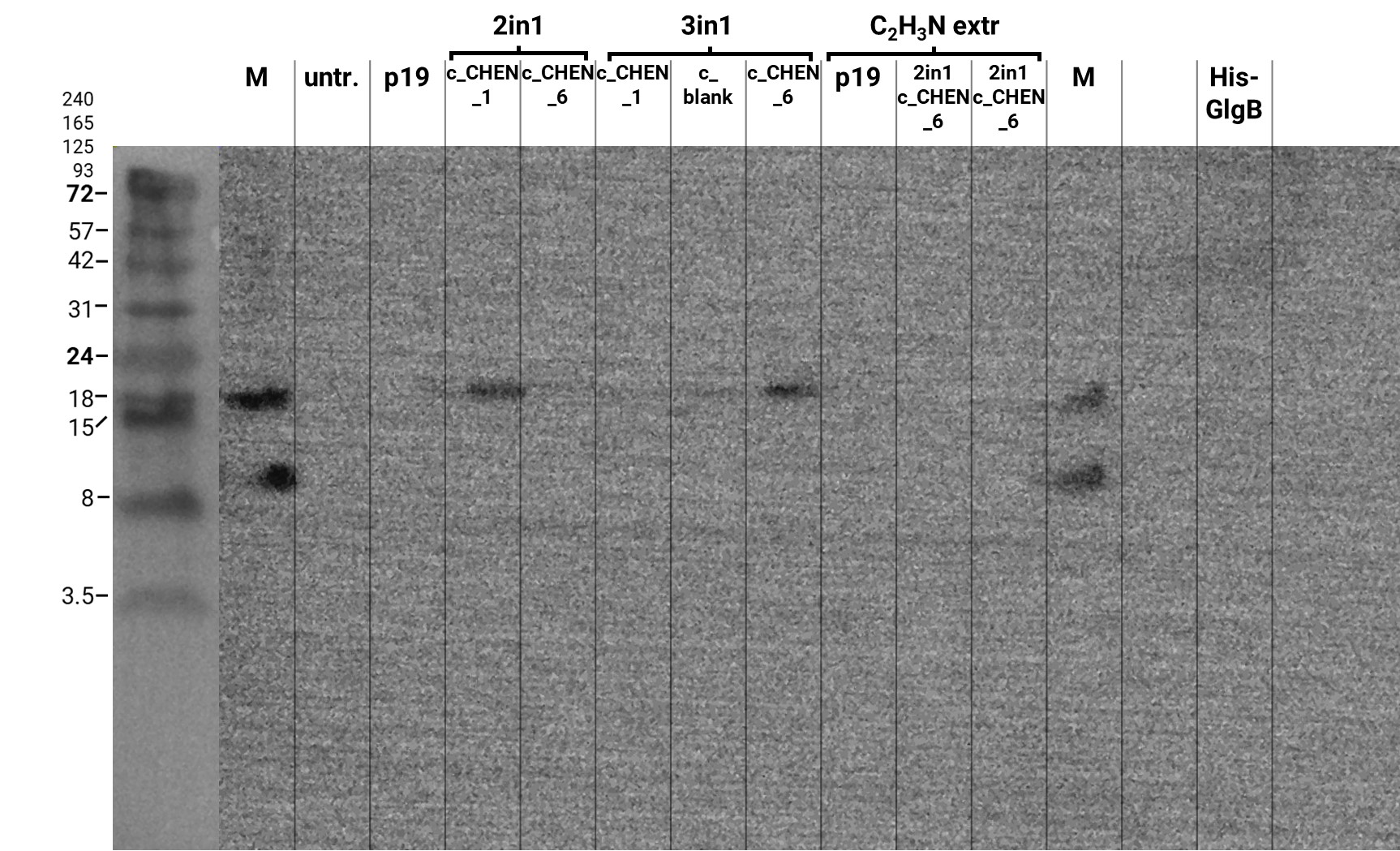
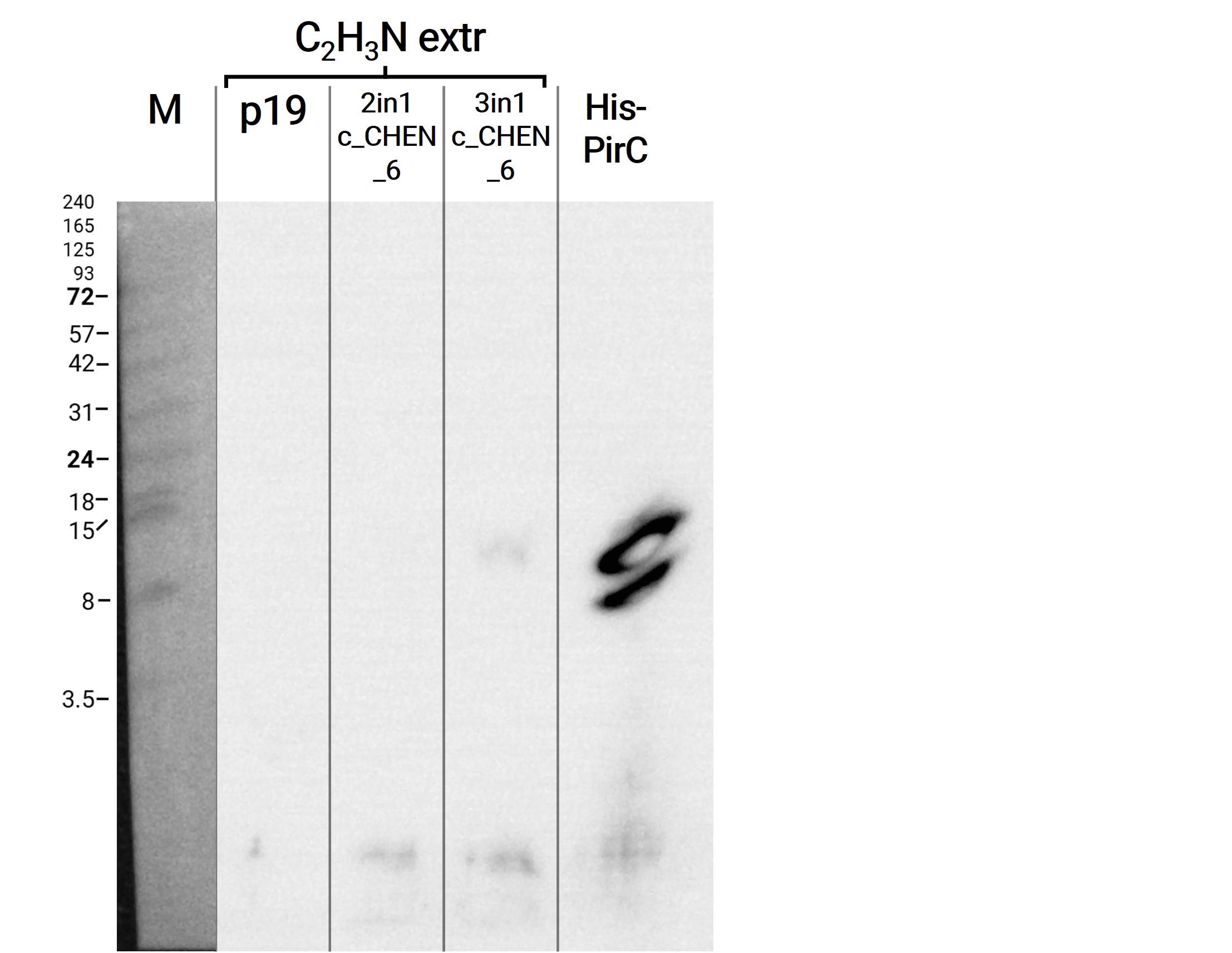
We then extracted leaves using an acidic-acetonitrile based buffer. For this method (adapted from Poon et al, 2018)4 we used dried leaf material and evaporated the extraction solvent in the last step. Prior to applying these samples onto the SDS-PAGE some of the extract was therefore solubilized in ddH2O and then mixed with 2X Lämmli buffer in equal ratio. On an anti-His-tag western blot we were able to detect some signal (figure 3). However, the molecular weight (MW) range (smaller than 3.5 kDa) in which the signal occurs does not correspond to anything we expect. Only in the lane of the construct 3in1 c_CHEN_6 (BBa_K3757006) a weak band can be observed at the MW of about 8 kDa, which would be around the expected size of our cyclotide construct. This indicates that the CtAEP1, which is co-expressed in construct 3in1 c_CHEN_6 and absent in construct 2in1 c_CHEN_6, could be expressed and actively cyclizing the AMP-cyclotide precursor. But as the signal is very low, this assumption would have to be verified by further experiments.
His-tag affinity purification
Another extraction was performed in HEPES buffer, whose composition was adjusted to the recommendations for the following His-purification via HisLink Protein Purification Resin (commercially available at Promega)5. Apart from the buffer we followed a protocol, which was shown to be suitable for the purification of plant peptides6. The steps of the purification were analyzed on an SDS-PAGE gel and by western blotting using an anti-His antibody. The first round of extraction and following purification was done with around 2 g of plant leaf tissue and a ratio of 1:2 plant mass:extraction buffer volume. In the extract, a total protein concentration between 0.6 to 1 mg/ml was detected. Afterwards purification via Ni-NTA was performed and the eluted fractions were then pooled and dialysed against HEPES buffer O.N. to remove the imidazole of the elution buffer. In this first experiment, no remarkable band was found in neither the Coomassie-stained SDS-PAGE gel (figure 4), nor the western blot (data not shown). Furthermore, the protein concentration in the eluate concentrated by centrifugation in protein concentrators (commercially available Amicon filters with a MWCO of 2 kDa) was under the detection limit of our protein quantification test and neither the crude extract nor the purified and concentrated protein showed any antimicrobial activity in our tests (data not shown here).

Conclusion
We were able to detect characteristic bands in the crude extracts of plants transfected with our constructs in an anti-His-tag western blot, which were not visible in the negative control crude extract (figure 2, figure 3). This confirms that the location of the His6-tag internally in a cyclotide loop does not or not entirely prevent its detection with an anti-His-tag antibody. This result is reinforced by the predicted structure of the His-tag in the grafted constructs, which shows it to be surface exposed. We were not able to purify our constructs with His-tag affinity purification. This could be due to the His-tag not being able to bind to the Ni-NTA resin in the purification cartridge we used. On the other hand, it might as well be that the expression levels of our constructs were simply too low to allow the purification of detectable amounts of our product.
References
1Craik, D. J., & Conibear, A. C. (2011). The chemistry of cyclotides. The Journal of Organic Chemistry, 76(12), 4805–4817. https://doi.org/10.1021/jo200520v
2Koehbach, J., Gani, J., Hilpert, K., & Craik, D. J. (2021). Comparison of a Short Linear Antimicrobial Peptide with Its Disulfide-Cyclized and Cyclotide-Grafted Variants against Clinically Relevant Pathogens. Microorganisms, 9(6), 1249. https://doi.org/10.3390/microorganisms9061249
3Jumper, John; Evans, Richard; Pritzel, Alexander; Green, Tim; Figurnov, Michael; Ronneberger, Olaf et al. (2021): Highly accurate protein structure prediction with AlphaFold. In: Nature. DOI: 10.1038/s41586-021-03819-2
4Poon, S., Harris, K. S., Jackson, M. A., McCorkelle, O. C., Gilding, E. K., Durek, T., van der Weerden, N. L., Craik, D. J., & Anderson, M. A. (2018). Co-expression of a cyclizing asparaginyl endopeptidase enables efficient production of cyclic peptides in planta. Journal of Experimental Botany, 69(3), 633–641. https://doi.org/10.1093/jxb/erx422
5Promega Corporation: HisLink Protein Purification Resin Technical Bulletin #TB327.
6Potula, H. H. Surya Kumar; Kathuria, Sonal Roy; Ghosh, A. K.; Maiti, T. K.; Dey, S. (2008): Transient expression, purification and characterization of bioactive human fibroblast growth factor 8b in tobacco plants. In: Transgenic research 17 (1), S. 19–32. DOI: 10.1007/s11248-007-9072-4.
| None |

