Here, we present the improvement of an iGEM-part for (a mostly faster,) easier and cheaper selection which suits our needs.
iGEM provides the universal acceptor plasmid BBa_P10500 for creating new PhytoBricks. This plasmid contained the lacZ-α part in the cloning sites for blue-white screening. As we wanted to establish Vibrio natriegens as a chassis for cloning, we investigated if the wild type strain is compatible with blue-white screening. Unfortunately, cloning using BBa_P10500 in V. natriegens shows no difference between colonies containing the lacZ-α dropout and those without.
To overcome this limitation and to enable fast and reliable cloning with V. natriegens, we decided to establish a new visualization method. Our improved part is a derivative of the iGEM BBa_P10500 containing a sfGFP dropout as fluorescent selection marker. In the pictures 2, you can see the wild type V. natriegens in comparison with the iGEM BBa_P10500 forming white colonies as well as bright green colonies containing the improved part BBa_K2560002. No differences between the wild type and the BBa_P10500 containing colonies are noticable. On the contrary, our new BBa_K2560002 part leads to a strong visual distinction to colonies which do not possess the sfGFP. In this way, we created a part for universal LVL0 cloning with a improved selection without the need of additional supplements like Xgal or IPTG.
Our part not only suits for V. natriegens but is convenient for the frequently used cloning host E. coli. As it can be seen in the picture 3, E. coli containing the sfGFP possess a considerable strong green colour even without the use of UV light and is just or even more distinguishable from the wild type as the lacZ containing blue colonies. By using our improved part BBa_K2560002 instead of the iGEM part BBa_P10500 work and money for the addition of the required supplements can be saved and the risk of not functional plates for selection is decreased. In our experiments, we were using 40 μg per Liter Xgal and 0,5 mM IPTG. Calculating with current prices, 100 plates supplemented with Xgal andIPTG costs about 80 dollar.

A: Colonies of wild type, B: white colonies containing BBa_P10500 and C: colonies with BBa_K2560002 resulting in green colonies.

A: Colonies of wild type, B: blue colonies containing BBa_P10500 and C: colonies with BBa_K2560002 resulting in green colonies.
By using our improved part for cloning, the detection of successfully ligated clones from non is feasible for strains which are not compatible with blue-white screening and therefor becomes more universal, faster and cheaper than before.
We proudly present the Marburg Collection, a novel golden-gate-based toolbox containing various parts that are compatible with the PhytoBrick system and MoClo. Compared to other bacterial toolboxes, the Marburg Collection shines with superior flexibility. We overcame the rigid paradigm of plasmid construction - thinking in fixed backbone and insert categories - by achieving complete de novo assembly of plasmids.
36 connectors facilitate flexible cloning of multigene constructs and even allow for the inversion of individual transcription units. Additionally, our connectors function as insulators to avoid undesired crosstalk.
The Marburg Collection contains 123 parts in total, including:
inducible promoters, reporters, fluorescence and epitope tags, oris, resistance cassettes and genome engineering tools. To increase the value of the Marburg Collection, we additionally provide detailed experimental characterization for V. natriegens and a supportive software. We aspire availability of our toolbox for future iGEM teams to empower accelerated progression in their ambitious projects.
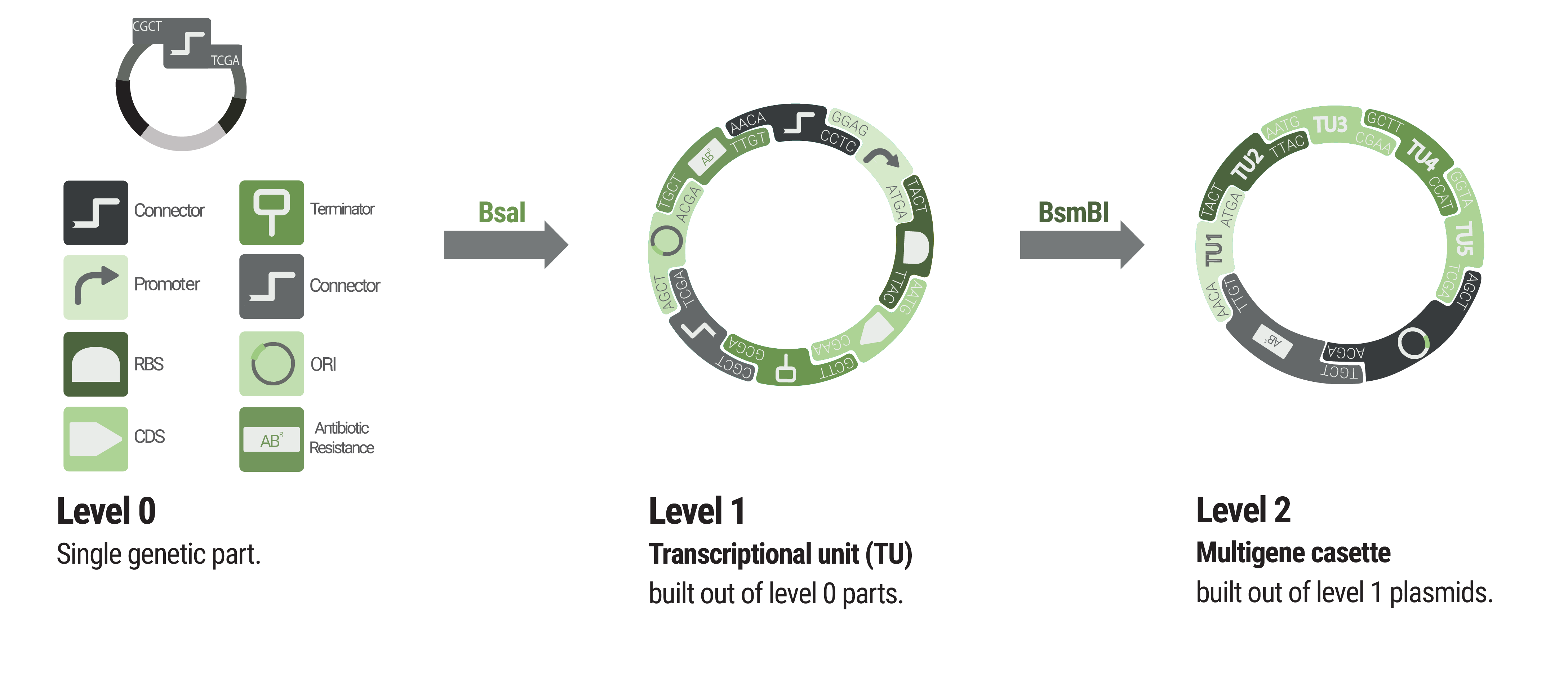
Basic building blocks like promoters or terminators are stored in level 0 plasmids. Parts from each category of our collection can be chosen to built level 1 plasmids harboring a single transcription unit. Up to five transcription units can be assembled into a level 2 plasmid.
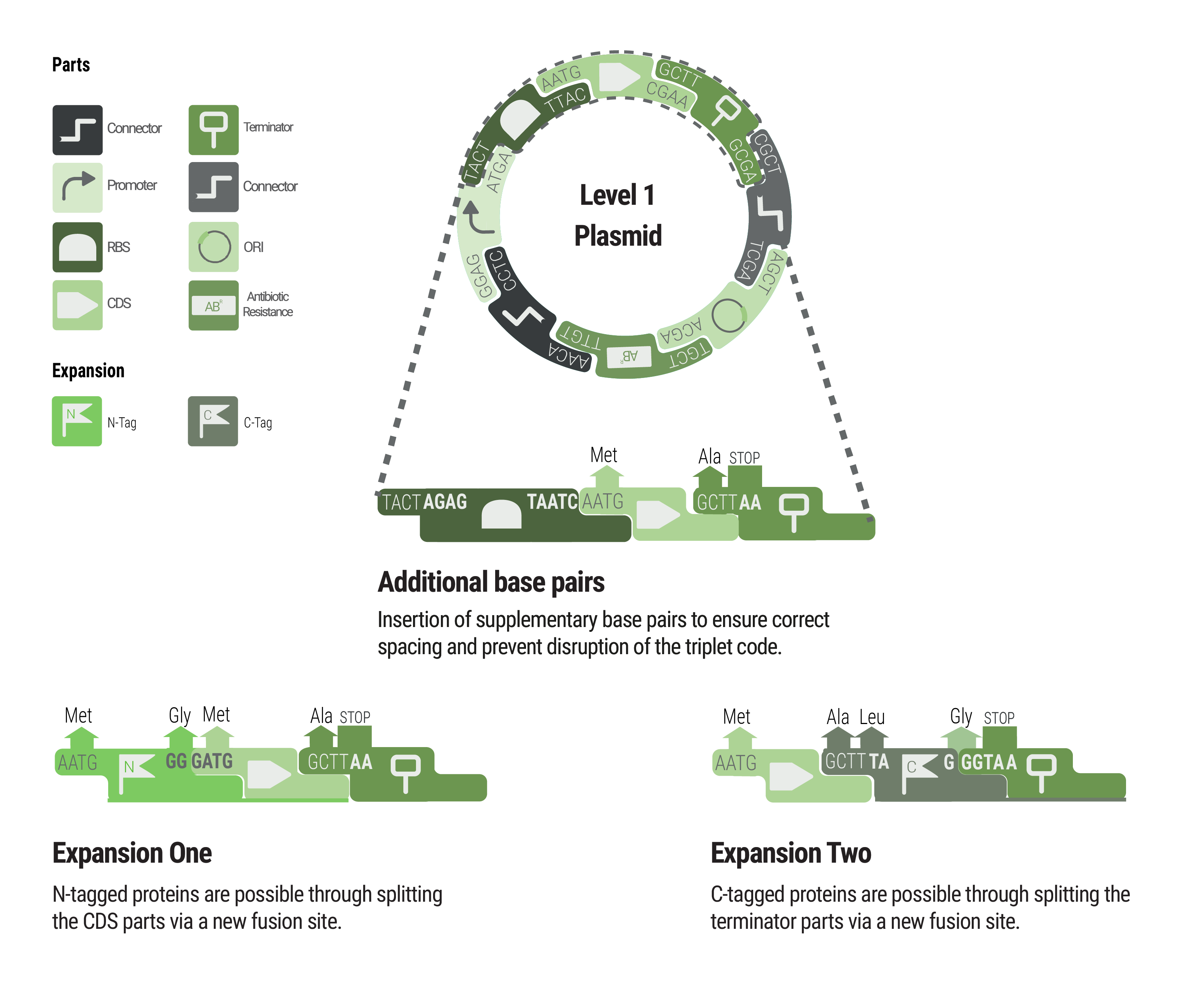
Between some parts, additional base pairs were integrated to ensure correct spacing and to maintain the triplet code. We expanded our toolbox by providing N- and C- terminal tags by creating novel fusions and splitting the CDS and terminator part, respectively.
Parts of the Marburg Toolbox

- K2560011 (5'Connector Dummy)
- K2560055
(1-6
Connector) - K2560065 (5'Con1)
- K2560066 (5'Con2)
- K2560067 (5'Con3)
- K2560068 (5'Con4)
- K2560069 (5'Con5)
- K2560075 (5'Con1
Short Res) - K2560076 (5'Con2
Short) - K2560077 (5'Con3
Short) - K2560078 (5'Con4
Short) - K2560079 (5'Con5
Short) - K2560095 (5'Con1 inv)
- K2560096 (5'Con2 inv)
- K2560097 (5'Con3 inv)
- K2560098 (5'Con4 inv)
- K2560099 (5'Con5 inv)
- K2560105 (5'Con5 inv
Ori) - K2560107 (5'Con1
Res)

- K2560007 (J23100)
- K2560009 (J23104)
- K2560014 (J23106)
- K2560015 (J23115)
- K2560017 (J23101)
- K2560018 (J23102)
- K2560019 (J23103)
- K2560020 (J23105)
- K2560021 (J23107)
- K2560022 (J23108)
- K2560023 (J23109)
- K2560024 (J23110)
- K2560025 (J23111)
- K2560026 (J23113)
- K2560027 (J23114)
- K2560028 (J23116)
- K2560029 (J23117)
- K2560030 (J23118)
- K2560031 (J23119)
- K2560123
(pTet) - K2560124 (pTrc)
- K2560131 (Promoter Dummy)

- K2560012 (3'Connector Dummy)
- K2560070 (3'Con1)
- K2560071 (3'Con2)
- K2560072 (3'Con3)
- K2560073 (3'Con4)
- K2560080 (3'Con5 Ori)
- K2560100 (3'Con1 inv
Short) - K2560101 (3'Con2 inv
Short) - K2560102 (3'Con3 inv
Short) - K2560103 (3'Con4 inv
Short) - K2560104 (3'Con5 inv
Short) - K2560106 (3'Con1 inv
Short Res) - K2560108 (3'Con1 inv)
- K2560109 (3'Con1 inv
Res) - K2560110 (3'Con2 inv)
- K2560111 (3'Con3 inv)
- K2560112 (3'Con4 inv)
- K2560113 (3'Con5 inv)

- K2560048 (Cam. Res. RFP)
- K2560056
(Kan. Res. (pSB3K3) RFP) - K2560057
(Kan. Res. (pSB3K3) GFP) - K2560058
(Tet. Res. (pSB3T5) RFP) - K2560059
(Tet. Res. (pSB3T5) GFP) - K2560125 (Carb. Res. RFP)
- K2560126 (Carb. Res. GFP)
- K2560127 (Carb. Res. into BBa_K2560002)
- K2560132 (Cam. Res. into BBa_K2560002)
- K2560133
(Kan. Res. into BBa_K2560002) - K2560134
(Tet. Res. into BBa_K2560002)
Tags and Entry Vectors







