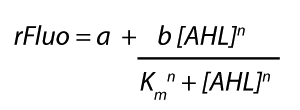Difference between revisions of "Part:BBa R0079:Experience"
(→Modeling crosstalk) |
(→Modeling crosstalk) |
||
| Line 50: | Line 50: | ||
|+ Parameters of HillFunction for crosstalk with Plas | |+ Parameters of HillFunction for crosstalk with Plas | ||
! | ! | ||
| − | |||
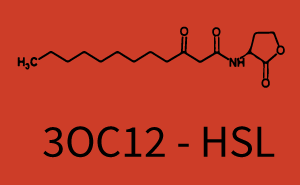
! 3OC12-HSL | ! 3OC12-HSL | ||
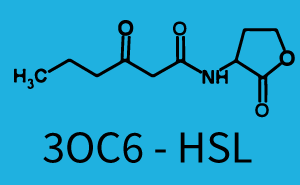
| + | ! 3OC6-HSL | ||
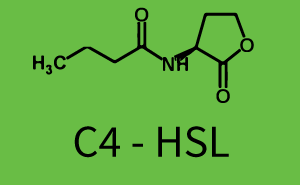
! C4-HSL | ! C4-HSL | ||
|- | |- | ||
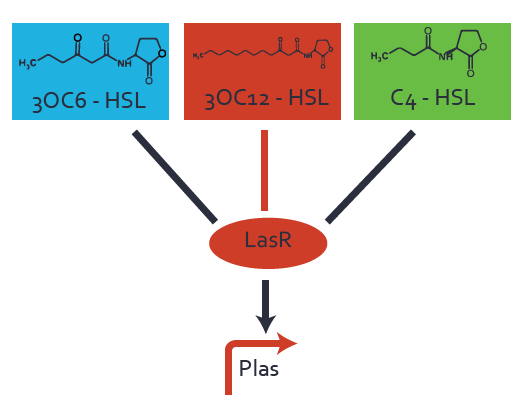
| − | ! | + | ! LasR |
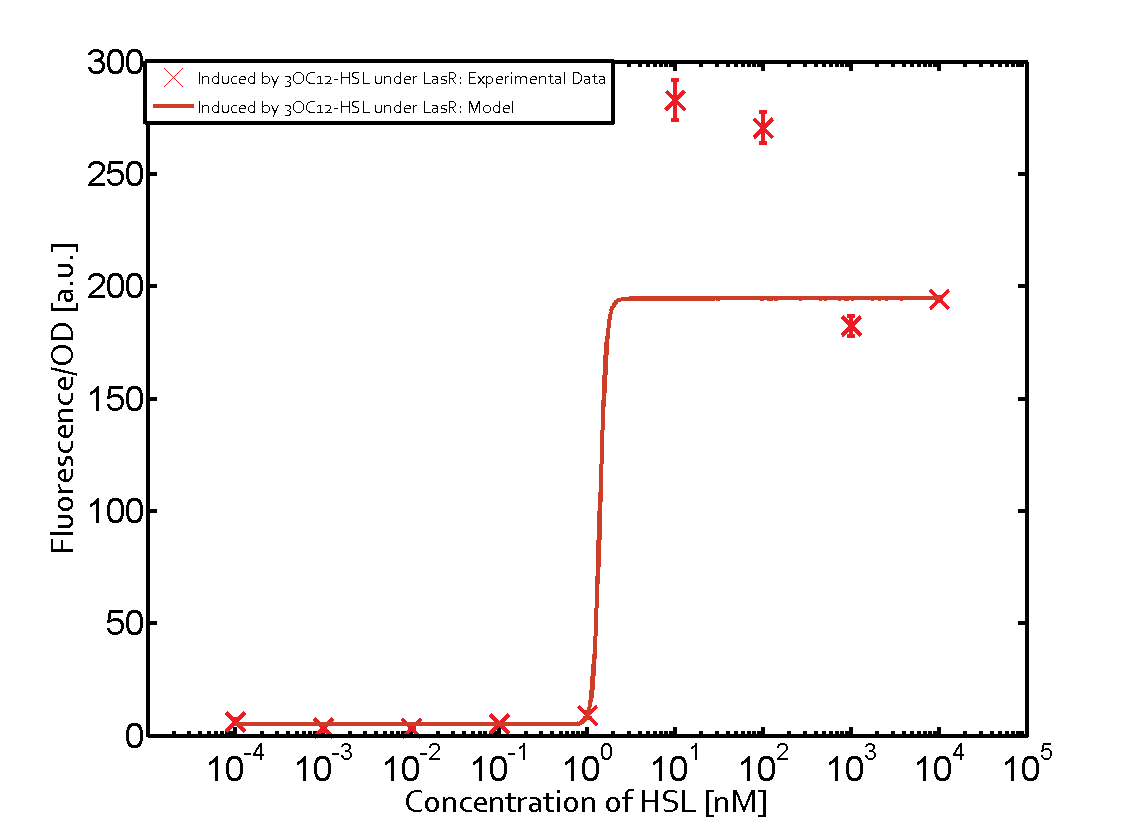
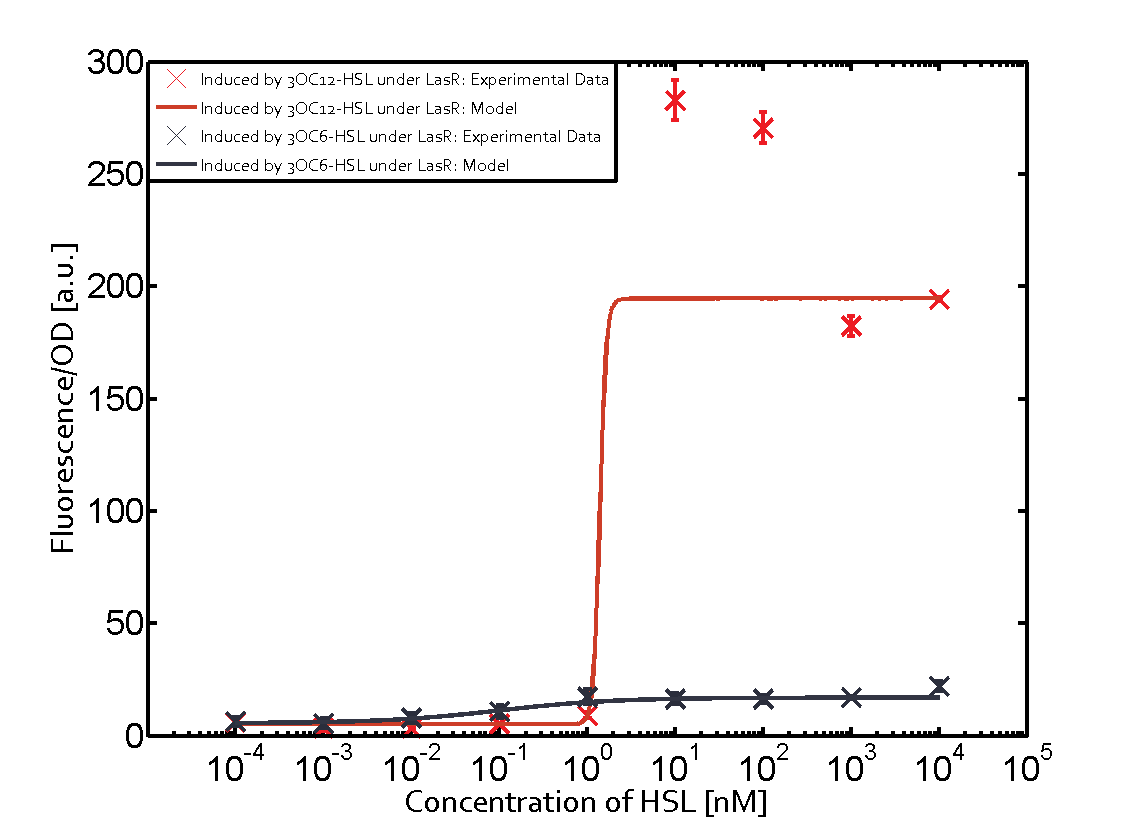
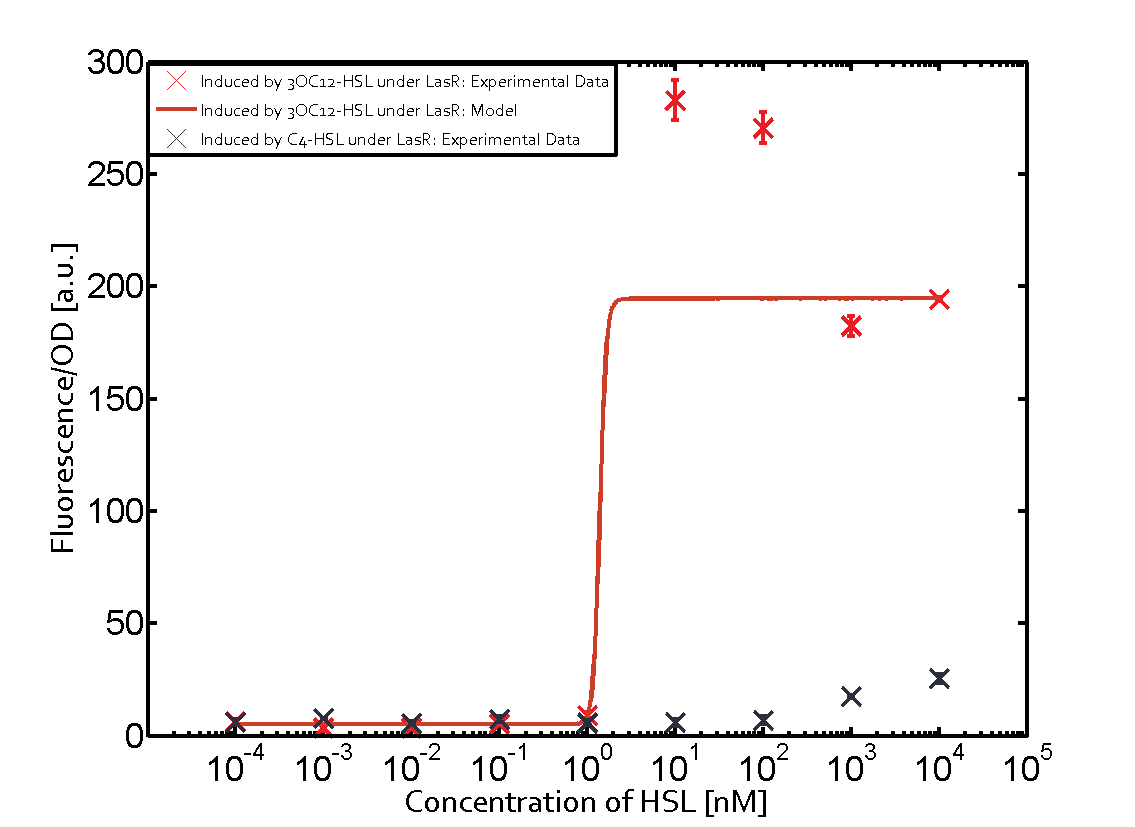
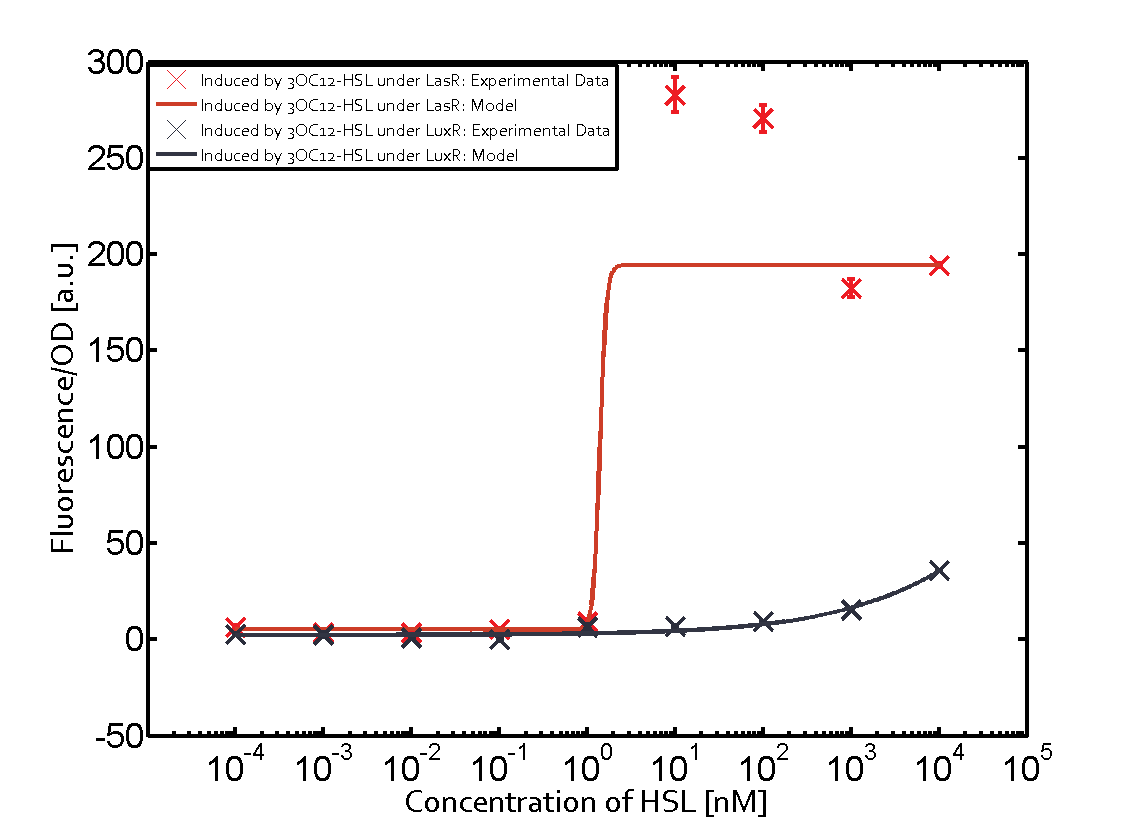
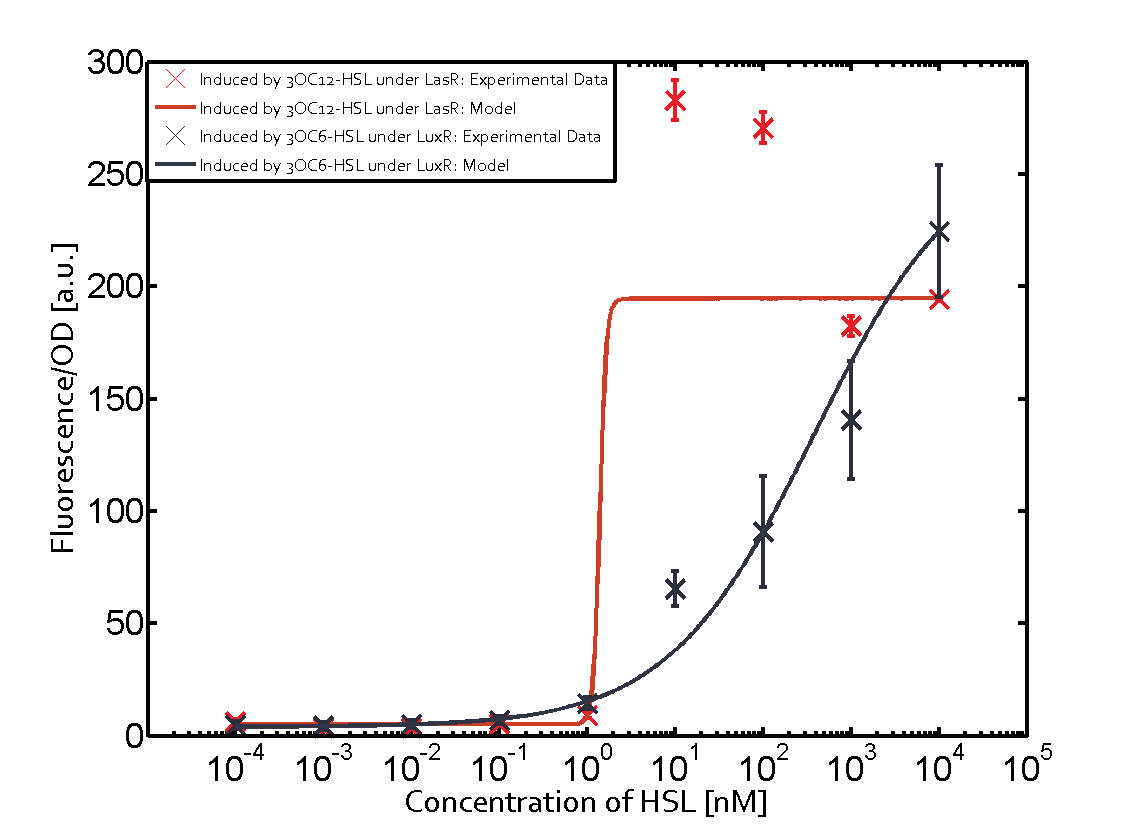
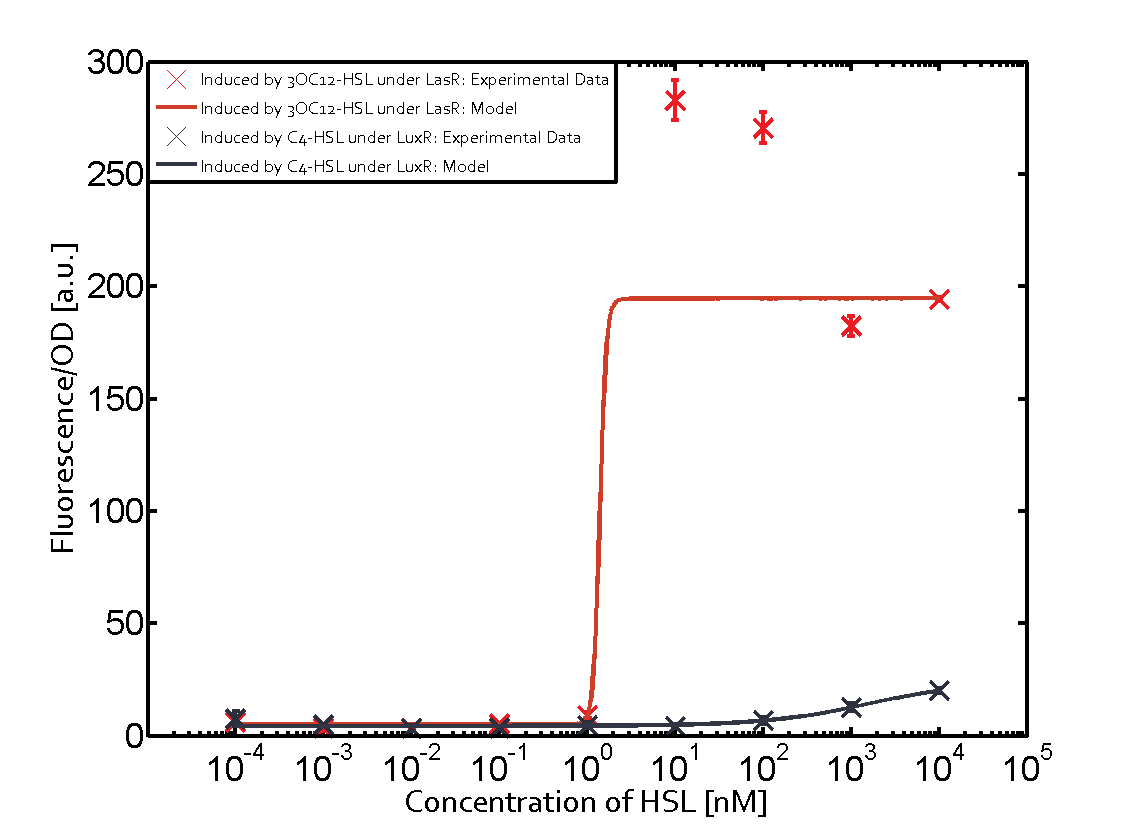
| − | | a = | + | | a = 5.274 (0, 15.51)<br> n = 11.67 (0, 3.763e8)<br> Km = 1.396 (0, 1.504e7)<br>b = 189 (162.5, 215.6)<br> |
| No crosstalk | | No crosstalk | ||
| No crosstalk | | No crosstalk | ||
|- | |- | ||
| − | ! | + | ! LuxR |
| No crosstalk | | No crosstalk | ||
| − | | a = | + | | a = 3.996 (0, 9.185)<br> n = 0.5198 (0.2879, 0.7517)<br> Km = 382.3 (0, 1206)<br>b = 261 (155.5, 366.5)<br> |
| No crosstalk | | No crosstalk | ||
|- | |- | ||
Revision as of 22:39, 23 October 2014
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_R0079
User Reviews
UNIQ76485783abe5f447-partinfo-00000000-QINU UNIQ76485783abe5f447-partinfo-00000001-QINU
|
••••
ETH Zurich 2014 |
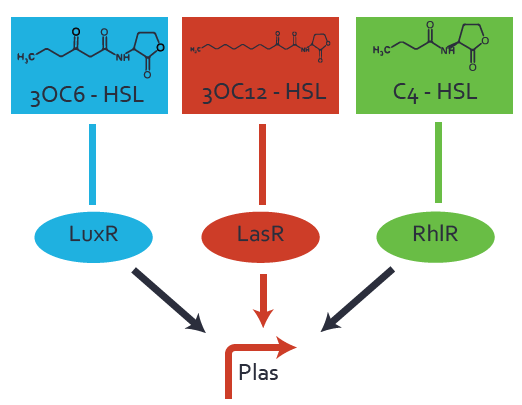
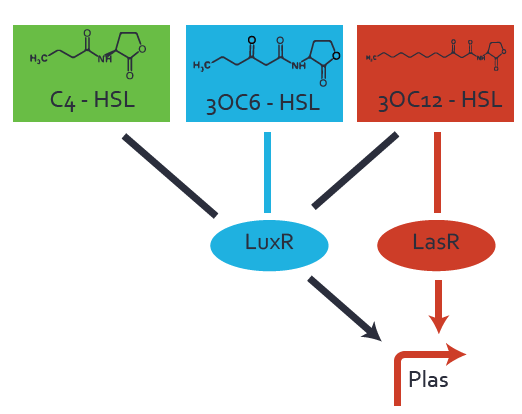
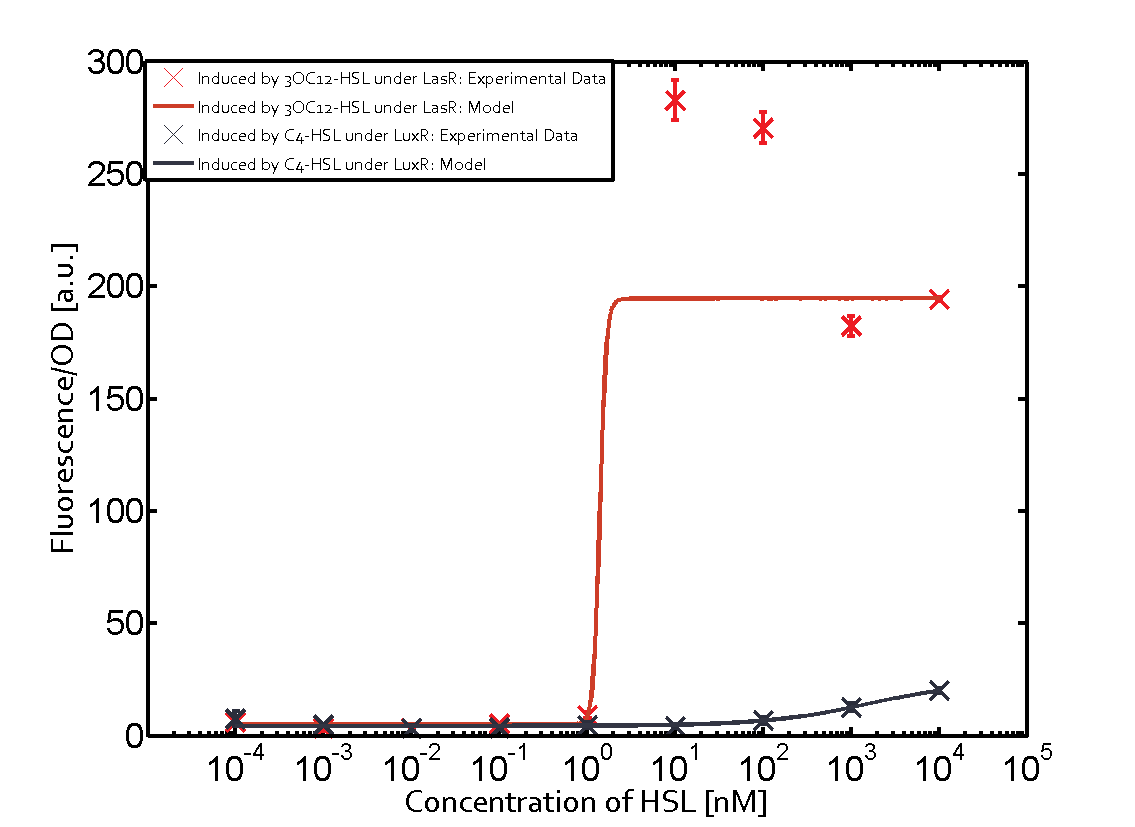
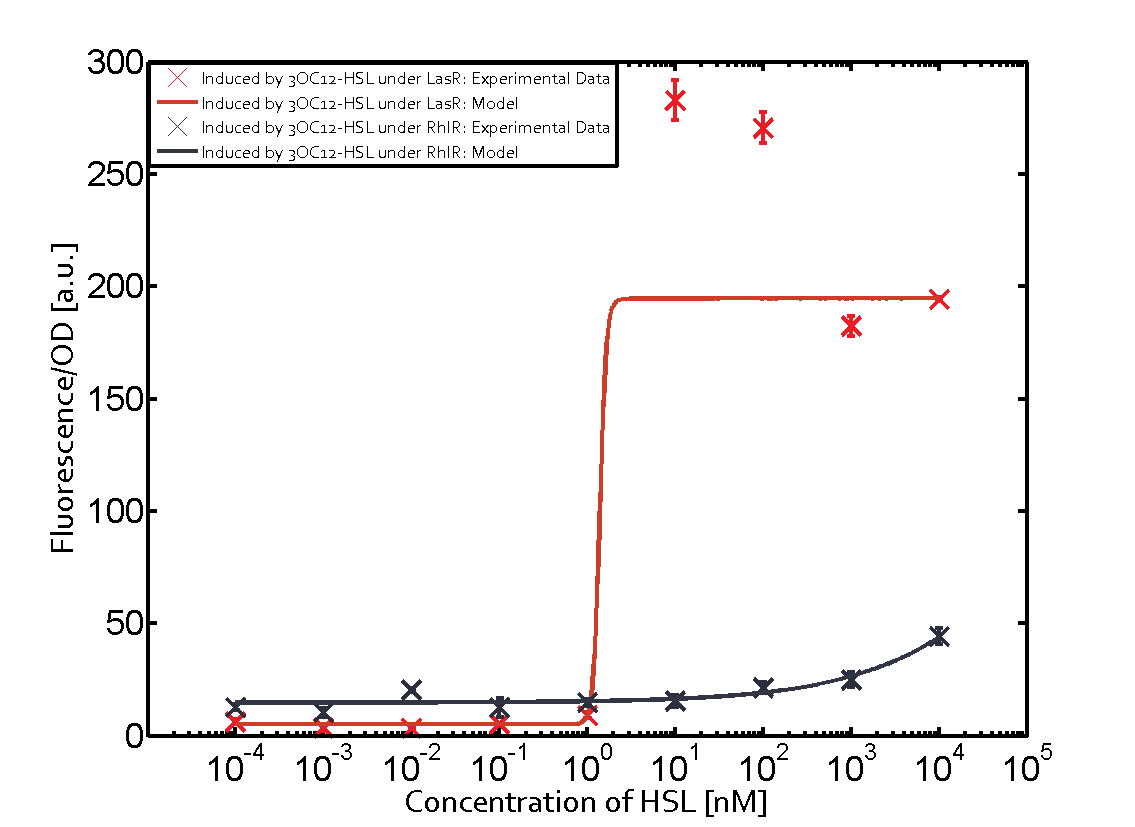
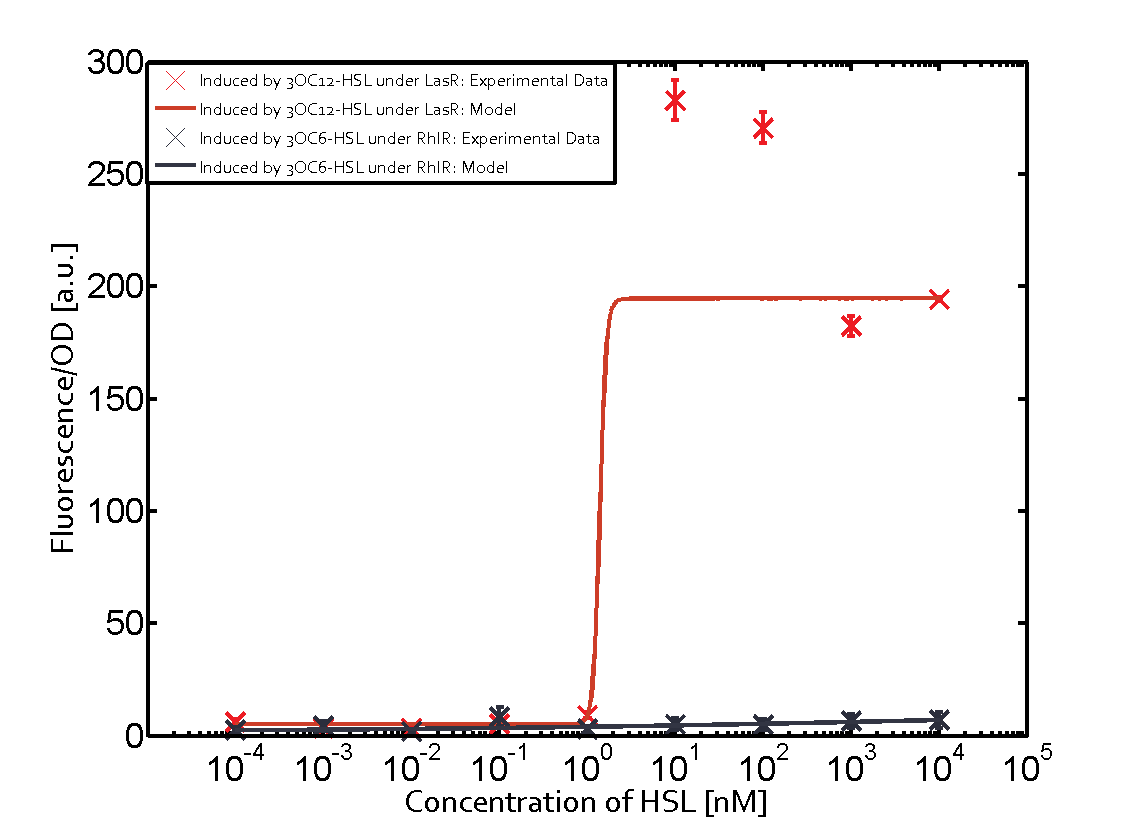
Characterization of two-order crosstalk on the promoterBackground informationSystem consideredModeling crosstalkEach experimental data set was fitted to an Hill function using the Least Absolute Residual method. The fitting of the graphs was performed using the following equation :
First-order crosstalkFirst Level crosstalk: LasR binds to different HSL and activates the promoterSecond Level crosstalk: other regulatory proteins, like LuxR, bind to their natural HSL substrate and activate the promoterSecond order crosstalk: Combination of both cross-talk levelsOther regulatory proteins, like LuxR, bind to different HSL and activates the promoter Results
| ||||||||||||||||||||||||||||||||||||
|
No review score entered. NYMU-Taipei 2009 |
|
|
No review score entered. Northwestern 2011 |
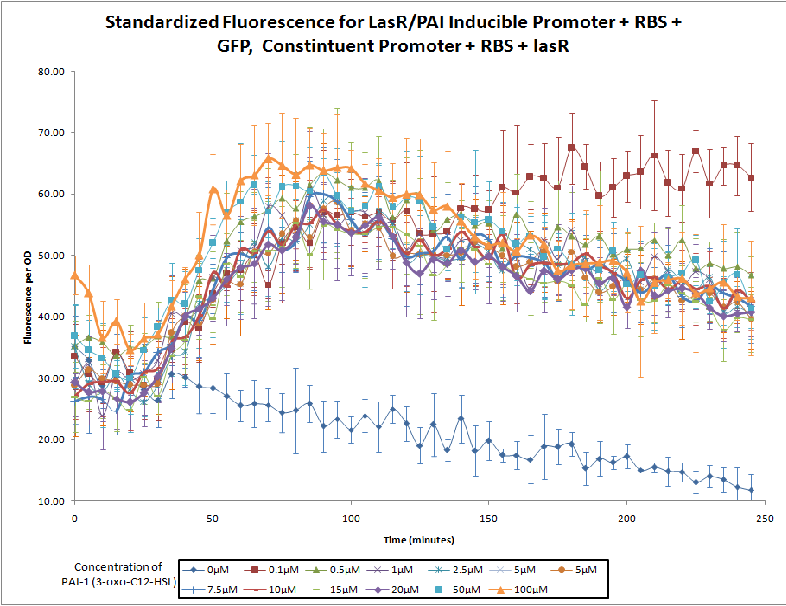
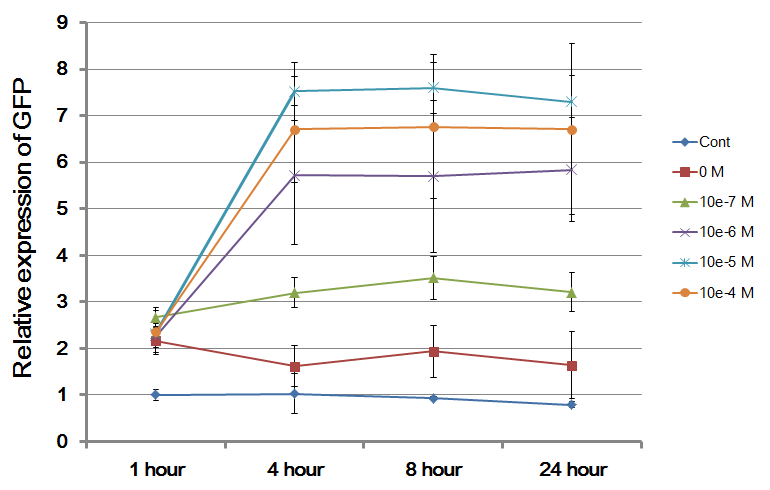
The Northwestern iGEM team used this part as a unit within our Pseudomonas Aeruginosa biosensor. When this LasR/PAI regulated promoter is induced at varying concentrations of PAI in the presence of excess LasR, we observed GFP fluorescence in accordance with the graph below. |
|};
|
•••
Tokyo-tech iGEM 2011 |
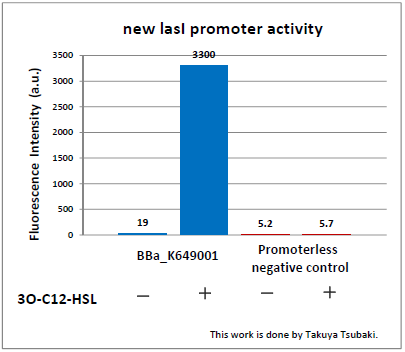
Judging from Northwestern iGEM 2011 team's data, in the presence of 3OC12-HSL, fluorescence intensity was about 3-fold higher than that in the absence of 3OC12-HSL.
|
|};
|
No review score entered. Tsinghua-A 2011 |
Tsinghua-A 2011 assembled E0840 (BBa_E0840) under the pLas promoter (BBa_R0079) that was contained into K574009 (BBa_K574009). We kept pSB1A2 as the scaffold vector.
|
|
•••••
iGEM Dundee 2014 |
Dundee iGEM 2014 used this lasB promoter region to build a composite part BBa_K1315009. This was designed as a biosensor for Pseudomonas aeruginosa AutoInducer-1 (PAI-1), which was to be used in a bio-electronic device to improve diagnostics for Cystic Fibrosis patients. Details of experimental work are logged on the experience page of BBa_K1315009. |