Part:BBa_K3781214
L1_sAP_RBD_GST, MocloMania Composite
This composite part contains the sAP secretion signal, a coding sequence for the SARS-CoV-2 receptor binding domain as well as a C-terminal GST-tag fused with a TEV protease recognition motif. The invidual basic parts are joined together by the MoClo connectors for B2/B3 and B4/B5.
L1_sAP_RBD_GST allows for transgenic expression of the SARS-CoV2 receptor binding domain in Leishmania tarentolae and secretion of the fusion protein into the culture medium. By concentrating the culture medium and running it over a gluthatione column, the GST-tagged RBD should be able to be purified.
level 1
size 1428 bp
antibiotic resistance in E. Coli ampicillin
plasmid backbone weird_plex
Data
We were able to successfully assemble this composite part into our L1 expression vector weird_plex and confirm the integrity of the resulting L1 construct via restriction digest and gel electrophoresis, see Figure 1. Along with subsequent sequencing, this verified the correct adaptation of the underlying basic parts to the MoClo cloning standard.
-
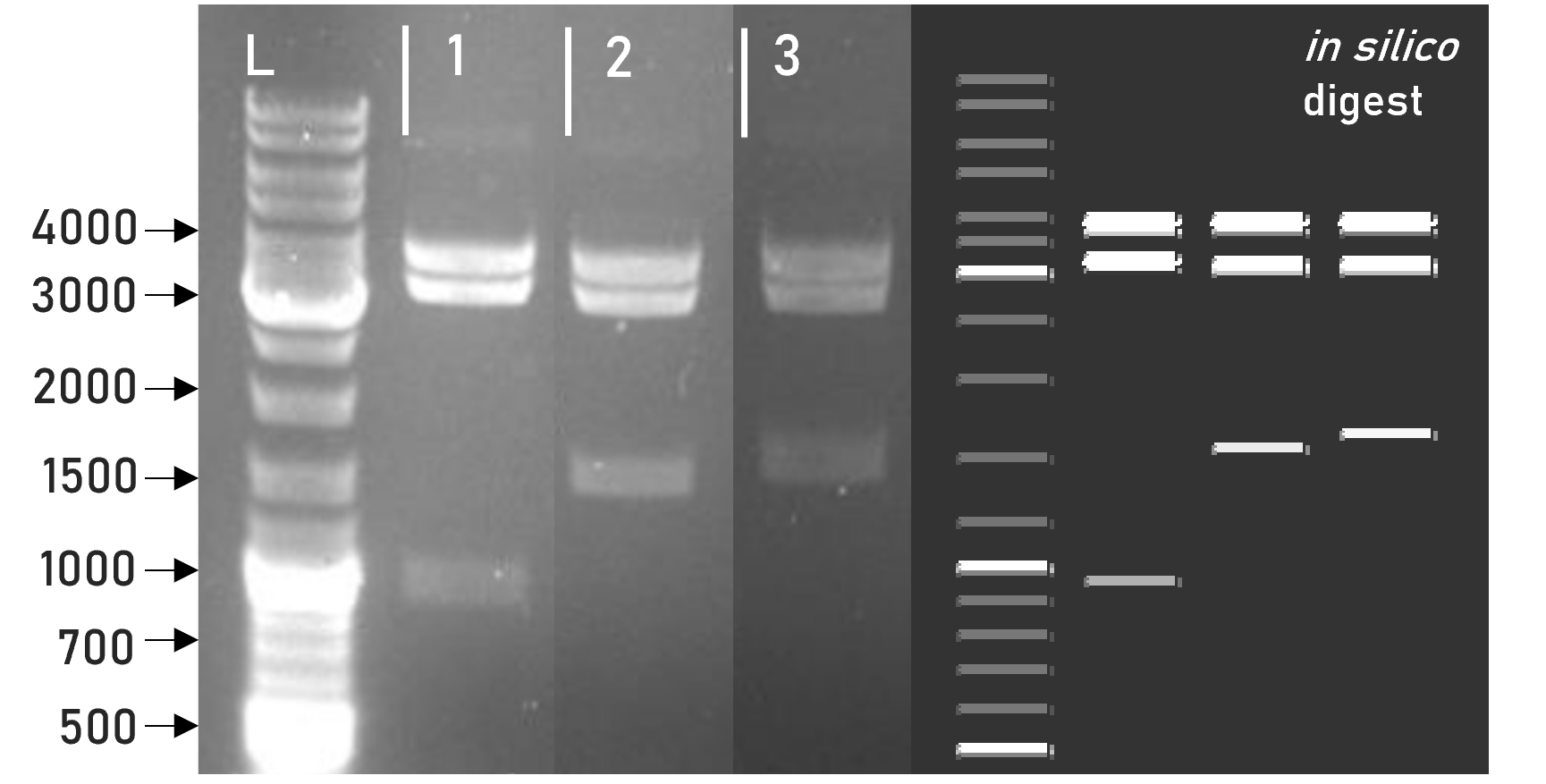 Figure 1 | Test digest of L1 constructs using HindIII
Figure 1 | Test digest of L1 constructs using HindIII
1 | L1_sAP_RBD_3xHA | 3792 + 3198 + 981 bp
2 | L1_sAP_RBD_GST | 3792 + 3198 + 1563 bp
3 | L1_sAP_mCerulean_GST | 3792 + 3198 + 1656 bp
L | Thermofischer GeneRuler Plus Ladder [bp]
Furthermore, L1_sAP_RBD_GST has been successfully transfected into Leishmania and recombinant protein expression could be observed via immunostaining on western blot, see Figure 2 and 3.
-
 Figure 2 | Immunoblot of L1 transfected Leishmania cell cultures | stained against RBD
Figure 2 | Immunoblot of L1 transfected Leishmania cell cultures | stained against RBD
1 | L1_sAP_RBD_Strep8His | 27.7 kDa
2 | L1_sAP_Myc_RBD_Strep8His | 30.2 kDa
3 | L1_sAP_RBD_GST | 51.9 kDa
n.c. | negative control | untransfected Leishmania culture
L | Thermofischer PageRuler Protein Ladder [kDa]
1. AB | ms anti-RBD | 1:2,000
2. AB | rb anti-ms HRP | 1:10,000 -
 Figure 3 | Immunoblot of L1 transfected Leishmania cell cultures | stained against GST
Figure 3 | Immunoblot of L1 transfected Leishmania cell cultures | stained against GST
1 | GST | 51.9 kDa
2 | L1_sAP_Strep_mCerulean_GST | 55.9 kDa
n.c. | negative control | Leishmania culture transfected with empty L1 expression vector
L | Thermofischer PageRuler Protein Ladder [kDa]
1. AB | gt anti-GST | 1:10,000
2. AB | rb anti-gt HRP | 1:2,000
Both immunostaining against RBD, see Figure 3, as well as against the GST-tag, see Figure 4, showed recombinant protein expression for cultures transfected with L1_sAP_RBD_GST. This verifies the cohesive nature of the underlying L1 construct and proves the structural integrity of the expressed fusion protein. In Figure 3, both the cell lysate as well as the culture supernatant of construct 3 | L1_sAP_RBD_GST display recombinant protein levels. In silico calculation predicts the full-length sAP_RBD_GST fusion protein to weigh about 55 kDa to which both upper bands can be attributed to. However, an additional, smaller band, running at approx. 27 kDa can be observed exclusively in the cell culture supernatant. Since this smaller band is also detected with anti-RBD antibodies, it is hypothesized that extracellular cleavage processes separate the RBD from its fusion tags after secretion of the fusion protein into the culture medium. For more information on this, please consult the sAP secretion tag part site.
In Figure 4, for both constructs the higher running band can be attributed to the full-length fusion protein. Here, smaller bands running at approx. 27 kDa in the cell culture supernatant can be observed as well. This time, tho, because it was stained against GST, the smaller band is assumed to correspond to cleaved molecular GST protein which is about 26 kDa in size. This explains why the smaller band can also be observed in the cell lysate, P and P+, especially for construct 1 | L1_sAP_RBD_GST, independently from secretion into the culture medium.
It also tells us that cleavage of the GST fusion tag is not only occurring extracellularly, but also intracellularly. The smeary bands in the supernatant of both constructs give rise to the idea that gradual degradation of the fusion protein might be occurring, maybe caused by uncoordinated proteolytic activity in the culture medium. This issue could be adversed by introducing protease inhibitor into the cell culture before protein harvest or analysis. We are working hard on optimizing expression levels and stability of fusion protein in order to make it even better accessible to downstream processing and increase yield. For more in-depth information on GST-tag cleavage, please refer to the L0_GST_B5 part page.
After western blot analysis, cultures transfected with L1_sAP_RBD_GST were introduced to downstream purification procedures. This further revealed the extent of GST-tag cleavage, but it also confirmed the binding of the RBD_GST fusion protein to gluthatione agarose resin during standard gravity-flow column purification, see Figure 5.
-
 Figure 5 | Immunoblot | L1_sAP_RBD_GST | after GST purification
Figure 5 | Immunoblot | L1_sAP_RBD_GST | after GST purification
1. AB | gt anti-GST | 1:10,000
2. AB | rb anti-goat HRP | 1:2,000
I Input | FT Flowthrough | W Wash | E Eluate | E.T Eluate, TCA precipitated
L.C | cell lysate of untransfected negative control
S.C | supernatant of untransfected negative control
S.A | supernatant after pelleting of ammonium precipitation
P.A | resuspended pellet of ammonium sulfate precipitation
GST | GST positive control | 26 kDa
RBD | RBD-GFP positive control | 52 kDa
L | Thermo Scientific PageRuler Prestained Protein Ladder [kDa]
As can be seen in Figure 5, all samples of L1_sAP_RBD_GST, before and after application to the gravity-flow column, display two distinct protein bands with very high intensity. The upper band, running just under 55 kDa, can be attributed to the full-length fusion protein with an in silico size of 51.9 kDa. Due to its visualisation with anti-GST antibody and its running size of about 26 kDa, the lower band is suspected to consist of singular GST protein. This band pattern showing up across all lanes suggests a possible cleavage of the GST-tag from its fusion protein in the culture supernatant. This of course negatively affects purification yield, since recombinant RBD cannot be captured. Since the GST attributed band is also visible in flowthrough, FT, and wash, W, only partial binding of GST to the gluthatione agarose resin seems to occur. The affinity and specifity of binding is influenced by a wide variety of factors. In order to improve column binding, we are currently working hard on testing different paramters for pH conditions, temperature, buffer concentrations and procedural set-up. Even though singular GST tag is detected in all lanes with virtually identical intensity, the full-length protein seems to display increasing concentrations in the different elution steps, E and E.T, suggesting at least a partially successful purification of target protein.
Despite very low purification yield, the samples resulting from GST-purification, along with other constructs carrying L0_RBD_B3_B4, were tested for functional activity of the receptor binding domain in an ACE2 binding assay. For this, we used human HEK 293T +ACE2 +TMPRSS2 (hek+) cells that we were given to us by our sponsor VectorBuilder. This specific cell line has a mutation that renders an overexpression of the RBD receptor angiotensin I converting enzyme 2, short ACE2. As negative control we employed HEK 293T cells that don’t express ACE2 (hek-). For a specific protocol for the conduction of this activity assay utilizing human cell culture, please refer to our Experiments page.
For the activity assay seen in Figure 6, one well of HEK-cells was incubated with L1_sAP_RBD_Strep8His and another well with weird_plex,for both of which the cell culture supernatant was concentrated through ammonium sulfate precipitation. One further well of HEK-cells were incubated with the purified L1_sAP_RBD_GST. HEK- -cells were incubated as negative controls.
-
 Figure 6 | Immunoblot | stained against ACE2 and RBD.
Figure 6 | Immunoblot | stained against ACE2 and RBD.
10% SDS-gel | 20 µL loaded per sample
A | stained with ms α-ACE2 | 1:1000
B | stained with ms α-RBD | 1:2000
1 | HEK-- cells incubated with 200 µL RBD_TEV_GST | purified
2 | HEK+-cells incubated with 1.5 mL RBD_Strep8His | concentrated through ammonium sulfate precipitation
3 | HEK+-cells incubated with 1.5 mL weird_plex | to rule out cross reaction with Leishmania supernatant
4 | HEK+- cells incubated with 200 µL RBD_TEV_GST | purified
5 | HEK--cells incubated with 1.5 mL RBD_Strep8His | concentrated through ammonium sulfate precipitation
6 | HEK+-cells only
7 | HEK-cells only
L | Thermo Scientific PageRuler Protein Ladder
For the fusion protein RBD_Strep8His on HEK+ cells, we can see a band at the expected height (27 kDa), which is a first indication of functionality of our protein RBD. Furthermore, the band is not present in the negative control (Hek--RBD-Strep8His) where we incubated the HEK--cells with the same sample. This proves that the band is not a cross reaction of the antibody with any other proteins of the HEK cells or Leishmania supernatant.
The purified RBD_GST shows no such band. This could be because the RBD got lost during purification, or because the GST tag, which is as big as the RBD itself, structurally interferes with ACE2 binding. The HEK-cells that were incubated with weird_plex show, as expected, no RBD.
The MocloMania collection
This L1 construct was assembled using basic parts from the MocloMania collection, the very first collection of genetic parts specifically designed and optimized for Modular Cloning assembly and recombinant protein expression in the protozoan parasite Leishmania tarentolae.
Are you trying to express complexly glycosylated proteins? Large antibody side chains? Human proteins that require accurate post-translational modification? Then Leishmania might be just the right organism for you! Leishmania tarentolae’s glycosylation patterns resemble those of human cells more closely than any other microbial expression host, while still delivering all the benefits of microbial production systems like easy transfection and cultivation.[1] So instead of relying on mammalian cell lines, try considering Leishmania as your new expression host of choice!
Our MocloMania collection will allow you to easily modify your protein of choice and make it suitable for downstream detection and purification procedures - all thanks to the help of Modular Cloning. This cloning system was first established by Weber et al. in 2011 and relies on the ability of type IIS restriction enzymes to cut DNA outside of their recognition sequence, hereby generating four nucleotide overhangs.[2] Every basic part in our collection is equipped with a specified set of overhangs that assign it to its designated position within the reading frame. These so-called cloning positions are labelled B2-B5 from upstream to downstream. By filling all positions with the basic parts of your choice, you can easily generate variable genetic constructs that code for the fusion protein of your desire.
We furthermore provide a specifically domesticated Leishmania expression vector, named weird_plex, which will package your fusion construct into a functional transcriptional unit that is optimized for high expression in Leishmania.
The best part? Because of the type IIS restriction properties and the specifity of the generated overhangs, restriction and ligation of your construct can all happen simultaneously in a simple one-step, one-pot reaction. This will safe you a lot of time and frustration in your cloning endeavours!
Do we have your attention? In the table below you can find some basic information on how our cloning system, along with most other MoClo systems, is set up. Please feel free to check out our wiki to find more information on Leishmania and Modular Cloning as well as to understand how the part that you are looking at integrates into our part collection. See you there!
| Level | What does this level contain? | antibiotic resistance | Enzyme used for ligation |
| L0 | The foundation to every MoClo construct which are basic genetic units, such as coding sequences, promoters, terminators | spectinomycin | BbsI |
| L1 | Several L0 parts assembled into a functional transcriptional unit, e.g. consisting of promoter, coding region and terminator | ampicillin | BsaI |
| L2 | Multiple transcriptional units added into one multi-gene construct, e.g. a protein of interest fused to a selection marker | kanamycin | BbsI |
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 598
Illegal PstI site found at 1381 - 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 598
Illegal PstI site found at 1381 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 536
Illegal XhoI site found at 6 - 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 598
Illegal PstI site found at 1381 - 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 598
Illegal PstI site found at 1381
Illegal NgoMIV site found at 684 - 1000COMPATIBLE WITH RFC[1000]
Reference Literature
- ↑ Langer T, Corvey C, Kroll K, Boscheinen O, Wendrich T, Dittrich W. Expression and purification of the extracellular domains of human glycoprotein VI (GPVI) and the receptor for advanced glycation end products (RAGE) from Rattus norvegicus in Leishmania tarentolae. Prep Biochem Biotechnol. 2017 Nov 26;47(10):1008-1015. doi: 10.1080/10826068.2017.1365252. Epub 2017 Aug 31. PMID: 28857681.
- ↑ Weber E, Engler C, Gruetzner R, Werner S, Marillonnet S (2011) A Modular Cloning System for Standardized Assembly of Multigene Constructs. PLoS ONE 6(2): e16765. https://doi.org/10.1371/journal.pone.0016765
| None |