Part:BBa_K3781005
sAP secretion tag, MocloMania B2
The sAP tag is a secretion signal peptide derived from the secretory protein secreted acid phosphatase 1 (sAP1) which was originally purified from Leishmania mexicana, a human pathogenic Leishmania strain.[1] The secretion of acid phosphatases makes the parasites more resistant to oxidative stress induced by the host’s immune cells.[2] The secretion signal peptide found in sAP1 mediates the exocytosis of the protein after its biosynthesis in the endoplasmatic reticulum (ER). This is extremely useful for recombinant protein production, since target proteins can be harvested directly from cell culture supernatant without the need for cell lysis. Since post-translational glycosylation of proteins happens within ER and Golgi apparatus, along the secretory pathway[3], the sAP1 signal peptide tag furthermore enables the production of glycosylated proteins in Leishmania.
The sequence to this part was directly derived from the commercial pLEXSY_I-blecherry3 plasmid, distributed by JenaBioscience, which was domesticated towards the MoClo system in order to work as a L1 expression vector for the MocloMania collection. Because this plasmid allows for either cytosolic or secretory protein production, the sAP secretion signal peptide was already included in the plasmid.[4] As part of the domestication process, we eliminated the sequence from the vector, but instead adapted it and turned it into a functional L0 basic part in our MocloMania collection. This way, switching between cytosolic and secretory protein expression is easily coordinated within a simple MoClo reaction.
It is important to note that since sAP is a secretion signal peptide, it only destines the fused protein to enter the secretory pathway. The signal peptide itself is cleaved off post-translationally before the actual exocytosis of the protein into the cell culture medium.[5]
size 2.3 kDa
function secretion tag
cloning position B2
plasmid backbone pAGM1276
Data
We were able to successfully clone this secretion tag into its respective L0 plasmid backbone and to confirm the integrity of the L0 construct via restriction digest and gel electrophoresis, see Figure 1. Since with our project we aimed to make use of Leishmania’s complex, human-like glycosylation capabilities that mostly rely on secretory protein expression, we included the sAP secretion tag into the vast majority of our assembled L1 constructs. This furthermore proved its correct adaptation towards MoClo assembly, see Figure 2.
-
 Figure 1 | Test digest of L0-sAP_B2 using BstEII and XhoI
Figure 1 | Test digest of L0-sAP_B2 using BstEII and XhoI
1 | pAGM1276 | 2842 bp
2 | L0_sAP_B2 | 1705 + 615 bp
L | Thermofischer GeneRuler Plus Ladder [bp] -
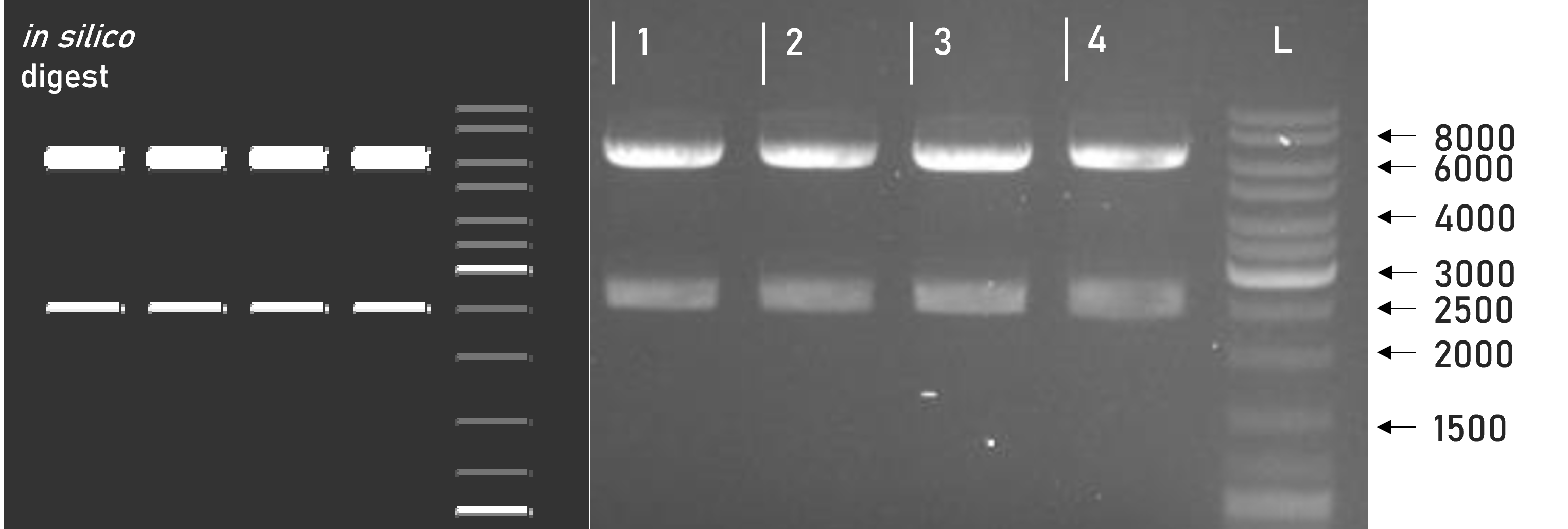 Figure 2 | Test digest of L1 constructs using SacI
Figure 2 | Test digest of L1 constructs using SacI
1 | L1_3xHA_RBD_mVenus | 6098 + 2515 bp
2 | L1_3xHA_RBD_mCerulean | 6098 + 2515 bp
3 | L1_sAP_RBD_mVenus | 6071 + 2515 bp
4 | L1_sAP_RBD_mCerulean | 6071 + 2515 bp
L | Thermofischer GeneRuler Plus Ladder [bp]
Almost all of the L1 constructs assembled with L0_sAP_B2 have been successfully transfected into Leishmania, showing recombinant protein expression after immunostaining on western blot, see Figures 3 and 4.
-
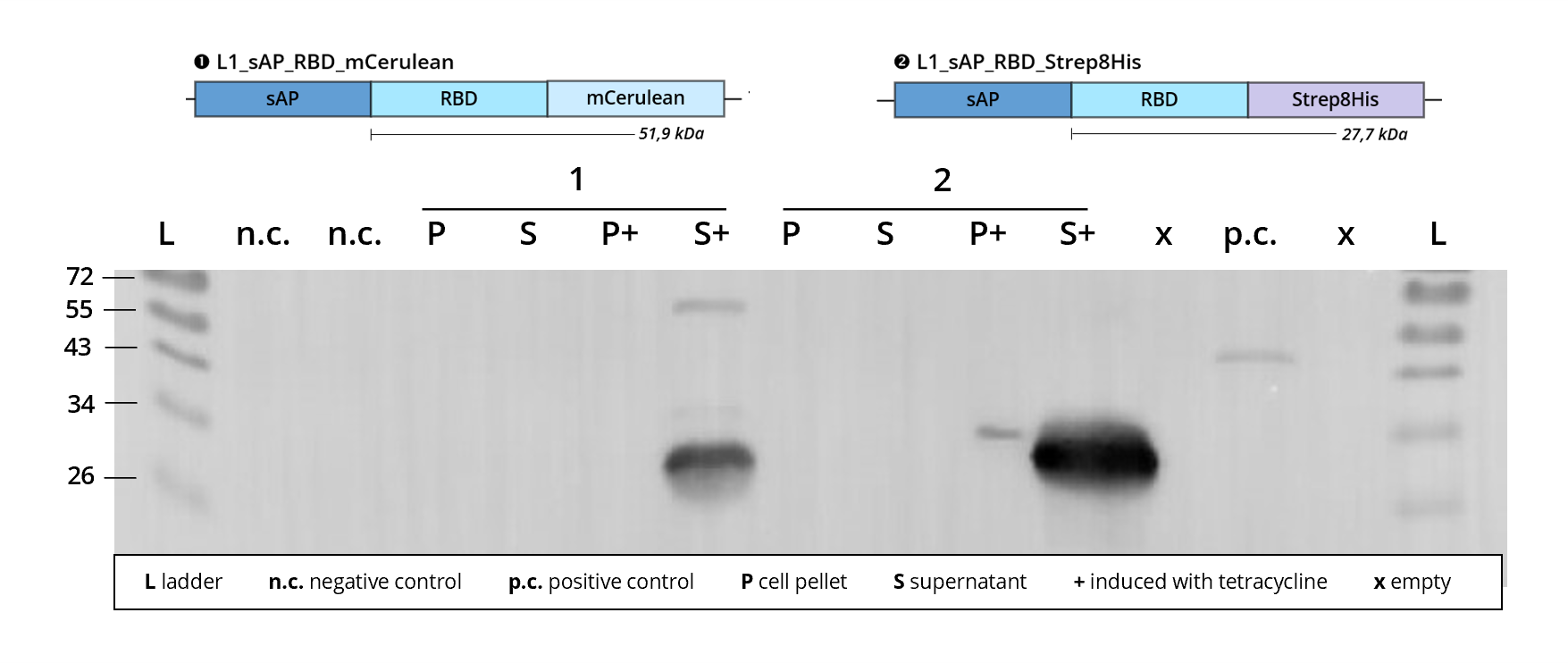 Figure 3 | Immunoblot of L1 transfected Leishmania | stained against RBD
Figure 3 | Immunoblot of L1 transfected Leishmania | stained against RBD
1 | L1_sAP_RBD_mCerulean | 51.9 kDa
2 | L1_sAP_RBD_Strep8His | 27.7 kDa
n.c. | negative control | untransfected Leishmania culture
p.c. | RBD-GFP | 54 kDa
L | Thermofischer PageRuler Protein Ladder [kDa]
1. AB | ms anti-RBD | 1:2,000
2. AB | rb anti-ms HRP | 1:10,000 -
 Figure 4 | Immunoblot of L1 transfected Leishmania cell cultures | stained against RBD
Figure 4 | Immunoblot of L1 transfected Leishmania cell cultures | stained against RBD
1 | L1_sAP_RBD_Strep8His | 27.7 kDa
2 | L1_sAP_Myc_RBD_Strep8His | 30.2 kDa
3 | L1_sAP_RBD_GST | 51.9 kDa
n.c. | negative control | untransfected Leishmania culture
L | Thermofischer PageRuler Protein Ladder [kDa]
1. AB | ms anti-RBD | 1:2,000
2. AB | rb anti-ms HRP | 1:10,000
Looking at Figure 3, construct 2 | L1_sAP_RBD_Strep8His shows protein expression in both the lysate, P+, as well as the supernatant, S+ of the induced culture when stained against the SARS-CoV-2 receptor binding domain. Judging from the signal intensity, protein levels within the supernatant by far exceed those within the lysate. This can be attributed to the sAP secretion tag mediating exocytosis of the fusion protein into the cell culture medium. Remaining bands within the lysate might be explained by recombinant protein still being intracellularly processed or getting stuck along the secretory pathway, e.g. during membrane passage. While the prominent band detected in the culture supernatant runs at the predicted size of 27 kDa, the band detected in the lysate is much thinner and runs at about 30 kDa. This size difference between intracellular and extracellular fusion protein might result from the cleavage of the sAP secretion tag (2.3 kDa) upon exocytosis</b>.
In construct 1 | L1_sAP_RBD_mCerulean, protein expression can be detected exclusively in the cell culture supernatant. In silico calculation estimates the size of the L1 constructs to be around 52 kDa. Taking into considerations size shifts caused by SDS PAGE deficiencies, the upper band can be attributed to this size. Yet, another smaller band, running at approx. 27 kDa appears on the blot. This phenomenon was observed on most of the western blots conducted throughout our experiments and always follows the same pattern. The lanes show upper bands that correspond to the theoretical size of the respective full-length fusion protein, but they are always accompanied by additional smaller bands, most prominently a band at 27 kDa that can be stained with anti-RBD antibodies.
In 90 % of cases, this additional band can be detected exclusively within the cell culture supernatant, but not in the cell lysate. This is particularly evident in Figure 4 where all three constructs show protein bands in the lysate as well as the supernatant, but only when it comes to the upper band attributed to the full-length fusion protein size. For construct 2 | L1_sAP_Myc_RBD_Strep8His this is 30 kDa, for construct 3 | L1_sAP_RBD_GST it's 52 kDa. The lysate samples of both display a thin band at the respective size and the supernatant samples' upper bands run only minimally higher (possibly due to added molecular weight of glycolysations attached). But they are both accompanied by a prominent lower band that runs at around 26 kDa. This corresponds to the molecular weight of the SARS-CoV-2 receptor binding domain.
All these observations lead us to hypothesize that something such as extracellular cleavage processes in the Leishmania medium must separate the SARS-CoV-2 receptor binding domain from its fusion tags after secretion of the fusion protein into the culture medium. One thing is for sure: the more RBD is detected as a single band, the fewer is still present within the desired fusion protein - meaning that some biological or technical issue is negatively influencing our protein yield. In order to find solutions to this problem, we are currently investigating possible modifications of our culture medium, looking for biochemical conditions that increase the stability of our target protein. As a first approach, adding protease inhibitor to the culture mix after transcription induction might prevent any unwanted proteolytic degradation processes in the culture supernatant.
When it comes to the sAP secretion tag itself, our western blot data collectively suggests that its integration into a L1 construct leads to exocytosis of recombinant protein into the culture supernatant, whereas for constructs without the signal peptide, such as L1_3xHA_RBD_mCerulean in Figure 5, recombinant protein can only be detected within the cell lysate. This verifies the functionality of L0_sAP_B2 as a secretion signal peptide and showcases its importance for production of recombinant secretory proteins.
-
 Figure 5 | Immunoblot | L1_sAP_RBD_GST | after GST-purification
Figure 5 | Immunoblot | L1_sAP_RBD_GST | after GST-purification
Ü | supernatant | Ü.C | concentrated supernatant
F | Flowthrough | W | Wash
E | Eluate | E.T | Eluate precipitated with TCA
L | Lysate | L/P | Lysate + cell debris
M | Thermofischer PageRuler Protein Ladder [kDa]
co-stained against both RBD
1. AB | ms anti-RBD | 1:2,000
2. AB | rb anti-ms HRP | 1:10,000
and against GST
1. AB | gt anti-GST | 1:10,000
2. AB | ms anti-gt HRP | 1:2,000
After protein expression analysis via western blot, our experimental goal includes the purification of recombinant protein from Leishmania cell culture in order to obtain pure, intact protein samples for downstream analysis and application. Including the sAP secretion signal into a L1 constructs sets the base for later purification of target protein straight from the culture medium. Since secretory protein concentrations are much more diluted than those of cytosolic protein in lysate, most purification procedures require concentration of the culture supernatant prior to application to the column material. Protein concentration can be achieved either by physical methods such as ultrafiltration in AMICONs or chemical methods such as ammonium sulfate precipitation. But can the protein translocation mediated by the sAP secretion tag actually be confirmed during protein purification? In order to capture possible faults in the expression system upon purification, we always applied concentrated supernatant as well as cell lysate of a transfected culture to separate columns in parallel, see Figure 5.
In this GST-purification we can see that even though recombinant RBD_GST can be detected in both the supernatant as well as the lysate purification, especially in the TCA precipitated eluate E.T, protein levels in the supernatant still exceed those in the lysate by far. This gives further evidence for the functionality of the sAP-mediated protein secretion signalling. In order to find out more about GST-purification and how to interpret the band pattern visible in Figure 5, please consult the L0_GST_B5 part page.
The MocloMania collection
This basic part is part of the MocloMania collection, the very first collection of genetic parts specifically designed and optimized for Modular Cloning assembly and recombinant protein expression in the protozoan parasite Leishmania tarentolae.
Are you trying to express complexly glycosylated proteins? Large antibody side chains? Human proteins that require accurate post-translational modification? Then Leishmania might be just the right organism for you! Leishmania tarentolae’s glycosylation patterns resemble those of human cells more closely than any other microbial expression host, while still delivering all the benefits of microbial production systems like easy transfection and cultivation.[6] So instead of relying on mammalian cell lines, try considering Leishmania as your new expression host of choice!
Our MocloMania collection will allow you to easily modify your protein of choice and make it suitable for downstream detection and purification procedures - all thanks to the help of Modular Cloning. This cloning system was first established by Weber et al. in 2011 and relies on the ability of type IIS restriction enzymes to cut DNA outside of their recognition sequence, hereby generating four nucleotide overhangs.[7] Every basic part in our collection is equipped with a specified set of overhangs that assign it to its designated position within the reading frame. These so-called cloning positions are labelled B2-B5 from upstream to downstream. By filling all positions with the basic parts of your choice, you can easily generate variable genetic constructs that code for the fusion protein of your desire.
We furthermore provide a specifically domesticated Leishmania expression vector, named weird_plex, which will package your fusion construct into a functional transcriptional unit that is optimized for high expression in Leishmania.
The best part? Because of the type IIS restriction properties and the specifity of the generated overhangs, restriction and ligation of your construct can all happen simultaneously in a simple one-step, one-pot reaction. This will safe you a lot of time and frustration in your cloning endeavours!
Do we have your attention? In the table below you can find some basic information on how our cloning system, along with most other MoClo systems, is set up. Please feel free to check out our wiki to find more information on Leishmania and Modular Cloning as well as to understand how the part that you are looking at integrates into our part collection. See you there!
| Level | What does this level contain? | antibiotic resistance | Enzyme used for ligation |
| L0 | The foundation to every MoClo construct which are basic genetic units, such as coding sequences, promoters, terminators | spectinomycin | BbsI |
| L1 | Several L0 parts assembled into a functional transcriptional unit, e.g. consisting of promoter, coding region and terminator | ampicillin | BsaI |
| L2 | Multiple transcriptional units added into one multi-gene construct, e.g. a protein of interest fused to a selection marker | kanamycin | BbsI |
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 6
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Reference Literature
- ↑ Ilg T, Stierhof YD, Etges R, Adrian M, Harbecke D, Overath P. Secreted acid phosphatase of Leishmania mexicana: a filamentous phosphoglycoprotein polymer. Proc Natl Acad Sci U S A. 1991;88(19):8774-8778. doi:10.1073/pnas.88.19.8774
- ↑ Anne C.S. Fernandes, Deivid C. Soares, Elvira M. Saraiva, José R. Meyer-Fernandes, Thaïs Souto-Padrón, Different secreted phosphatase activities in Leishmania amazonensis, FEMS Microbiology Letters, Volume 340, Issue 2, March 2013, Pages 117–128, https://doi.org/10.1111/1574-6968.12080
- ↑ Schjoldager, K.T., Narimatsu, Y., Joshi, H.J. et al. Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Biol 21, 729–749 (2020). https://doi.org/10.1038/s41580-020-00294-x
- ↑ https://www.jenabioscience.com/files/jenabioscience/datasheet_extern/EGE-1410.pdf, pages 9, 18, last visited 10/17/21, 11:00 ECT
- ↑ Klatt S, Konthur Z. Secretory signal peptide modification for optimized antibody-fragment expression-secretion in Leishmania tarentolae. Microb Cell Fact. 2012;11:97. Published 2012 Jul 25. doi:10.1186/1475-2859-11-97
- ↑ Langer T, Corvey C, Kroll K, Boscheinen O, Wendrich T, Dittrich W. Expression and purification of the extracellular domains of human glycoprotein VI (GPVI) and the receptor for advanced glycation end products (RAGE) from Rattus norvegicus in Leishmania tarentolae. Prep Biochem Biotechnol. 2017 Nov 26;47(10):1008-1015. doi: 10.1080/10826068.2017.1365252. Epub 2017 Aug 31. PMID: 28857681.
- ↑ Weber E, Engler C, Gruetzner R, Werner S, Marillonnet S (2011) A Modular Cloning System for Standardized Assembly of Multigene Constructs. PLoS ONE 6(2): e16765. https://doi.org/10.1371/journal.pone.0016765
| biology | Leishmania mexicana |
| function | secretion signal peptide |