
1 Registry Star

Signalling
Part:BBa_F2620:Specificity
Designed by: Barry Canton [bcanton@mit.edu] and Anna Labno [labnoa@mit.edu] Group: iGEM04_MIT (2004-08-09)
Specificity describes the ability of a device to distinguish between its cognate input and other similar inputs. There are many AHL molecules with a structure similar to 3OC6HSL. Some of these might be used in a system alongside 3OC6HSL and BBa_F2620. Hence, it is necessary to characterize to what extent, if any, BBa_F2620 responds to alternate AHL molecules. Please see the part experience page for a general description of our characterization method.
Data
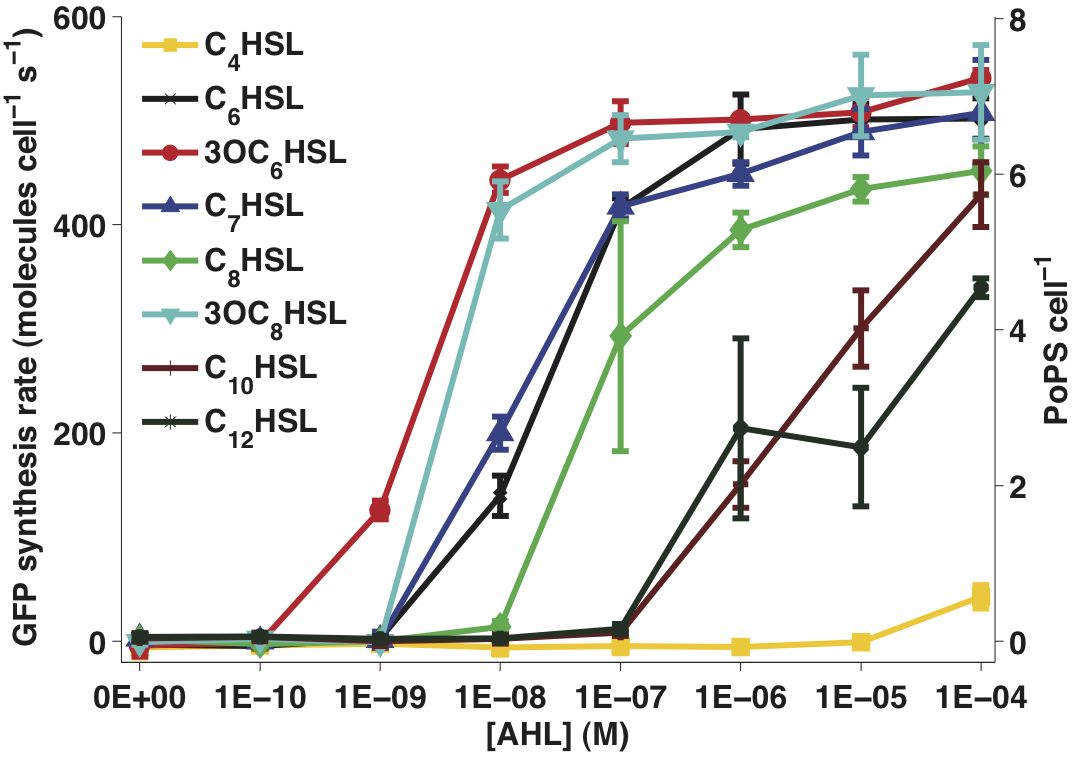
Figure 1 - Transfer function of BBa_F2620 for 8 different input molecules. The error bars represent 95% confidence intervals measured over three replicates.
Protocol
- Two cultures, one MG1655 bearing BBa_T9002 and one of MG1655 bearing the BBa_T9002 mutant lacking a GFP expression device (see stability section) were prepared as described in steps 1 – 3 of the transfer function section above. However, in this case the overnight cultures were diluted into 20 ml of fresh medium in a 200 ml flask and shaken at 220 rpm during growth.
- Three of the eight AHL variants (see table below) were preloaded into a flat-bottom 96 well plate to eight different final concentrations (0, 1E-10, 1E-9, 1E-8, 1E-7, 1E-6, 1E-5 M, and 1E-4 M). Three wells were each filled with 200 μl of media to measure the absorbance background. Three further wells were filled with 200 µl of the mutant BBa_T9002 culture to measure the fluorescent background.
- Seventy-two 200 µl aliquots of the BBa_T9002 culture were transferred to the plate. Three replicate wells were filled for each concentration of each AHL.
- The plate was incubated in a [http://openwetware.org/wiki/Endy:Victor3_plate_reader Wallac Victor3 multi-well fluorimeter] at 37°C and assayed with an automatically repeating protocol of absorbance measurements, fluorescence readings, and shaking (as described in the transfer function protocol). Time between repeated measurements was 2 min and 21 s.
- Steps 1 through 4 were repeated once with three more of the AHL variants and again with the final two AHL variants. The time between repeated measurements was kept fixed in each case.
- Data processing is described here. In Figure 1, snapshot transfer functions are plotted for each AHL variant at the 60 min time point similar to the transfer function experiment. The error bars represent the 95% confidence interval for the mean of the three replicate wells of each measurement.
| Full Name
|
Molecule abbreviation
|
Species
|
Notes
|
Source
|
Images (from Sigma Aldrich)
|
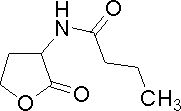
| Butanoyl-homoserine lactone
|
C4HSL
|
P. aeruginosa
|
|
[http://www.sigmaaldrich.com/catalog/search/ProductDetail/FLUKA/09945 Sigma Aldrich (#09945)]
|
|
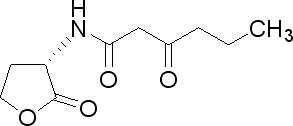
| 3-oxohexanoyl-homoserine lactone
|
3OC6HSL
|
V. fischeri
|
Lux system signaling molecule
|
[http://www.sigmaaldrich.com/catalog/search/ProductDetail/SIGMA/K3007 Sigma Aldrich (#K3007)]
|
|
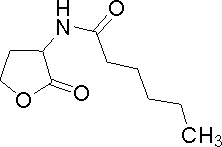
| Hexanoyl-homoserine lactone
|
C6HSL
|
C. violaceum
|
Very similar to 3OC6HSL
|
[http://www.sigmaaldrich.com/catalog/search/ProductDetail/FLUKA/09926 Sigma Aldrich (#09926)]
|
|
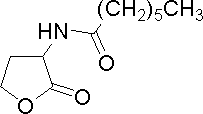
| Heptanoyl-homoserine lactone
|
C7HSL
|
E. psidii R. IBSBF 435T
|
|
[http://www.sigmaaldrich.com/catalog/search/ProductDetail/FLUKA/10939 Sigma Aldrich (#10939)]
|
|
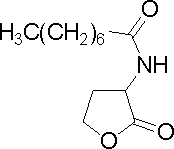
| Octanoyl-homoserine lactone
|
C8HSL
|
B. cepacia, V. fischeri
|
|
[http://www.sigmaaldrich.com/catalog/search/ProductDetail/FLUKA/10940 Sigma Aldrich (#10940)]
|
|
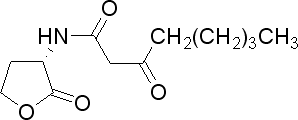
| 3-oxoctanoyl-homoserine lactone
|
3OC8HSL
|
A. tumefaciens
|
|
[http://www.sigmaaldrich.com/catalog/search/ProductDetail/SIGMA/O1764 Sigma Aldrich (#O1764)]
|
|
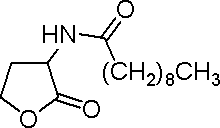
| Decanoyl-homoserine lactone
|
C10HSL
|
B. pseudomallei
|
|
[http://www.sigmaaldrich.com/catalog/search/ProductDetail/FLUKA/17248 Sigma Aldrich (#17248)]
|
|
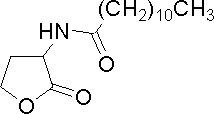
| Dodecanoyl-homoserine lactone
|
C12HSL
|
Synthetic
|
|
[http://www.sigmaaldrich.com/catalog/search/ProductDetail/FLUKA/17247 Sigma Aldrich (#17247)]
|
|








 1 Registry Star
1 Registry Star