Help:Assembly
Parts | Plasmid Backbones | BioBrick Prefix and Suffix | Standards | Assembly Standards | Assembly Methods
- Registry Help Pages:
- TOC
- At-a-Glance
- FAQ
What is Assembly
Assembly is the process by which part samples (belonging to the same assembly standard (RFC)) are connected to one another. Assembling two basic parts always results in a new, larger composite part that can be used in future assemblies. Assembly allows for the creation of parts that are longer and more complex in function, while still maintaining the format of the assembly standard. This idempotent assembly means that any newly composed part will adhere to its assembly standard without need for manipulation, and can be used in future assemblies without issue.
Assembly in relation too...
- Assembly standards define assembly through the use of a prefix and suffix, which flank the beginning and end of a part sample, respectively. The prefix and suffix contain restriction sites, which when used with specific restriction enzymes (cutting) and DNA ligase (connecting), allow part samples to be assembled together forming a new part. This new part sample will maintain the same prefix and suffix as its "parents" and contain a scar, where the cut and re-ligated restriction sites were stitched together. Note: There are also scarless assembly methods, which allow for the assembly of parts without the traditional use of the prefix and suffix restriction sites. The Registry mainly uses the following assembly standards:
- Plasmid Backbone propagates a sample of a part, located in between the prefix and suffix of the plasmid backbone. As such the plasmid backbone dictates the assembly standard and assembly of that part sample.
- Part - A part is compatible with an assembly standard, as long as its sequence meets the requirements of said standard; this means that the part does not have any internal restriction sites that would interfere with the assembly, also known as illegal restriction sites. It is important to remember that a part does not include the prefix and suffix as defined by the assembly standard.
Registry Supported Assembly Methods
Choose your assembly method, support the standard
Choose your assembly method and assemble your parts with a variety of assembly techniques. You can use 3A Assembly, Type IIS, synthesis, Gibson, and more.
Support the standard. Although users can add parts to the Registry that adhere to other assembly standards, BioBrick RFC[10] and Type IIS are the Registry's current de facto standards; all parts on the Registry that will be considered for the iGEM competition (medals, awards, etc.) must be assembly compatible for BioBrick or Type IIS.
In 2019, sample submission is no longer required. However, ensuring your parts are assembly compatible allows the Registry to synthesize your parts in BioBrick and/or Type IIS format. Registry members can easily and reliably use and assemble these parts in the future.
Restriction Enzyme Assembly
3A Assembly
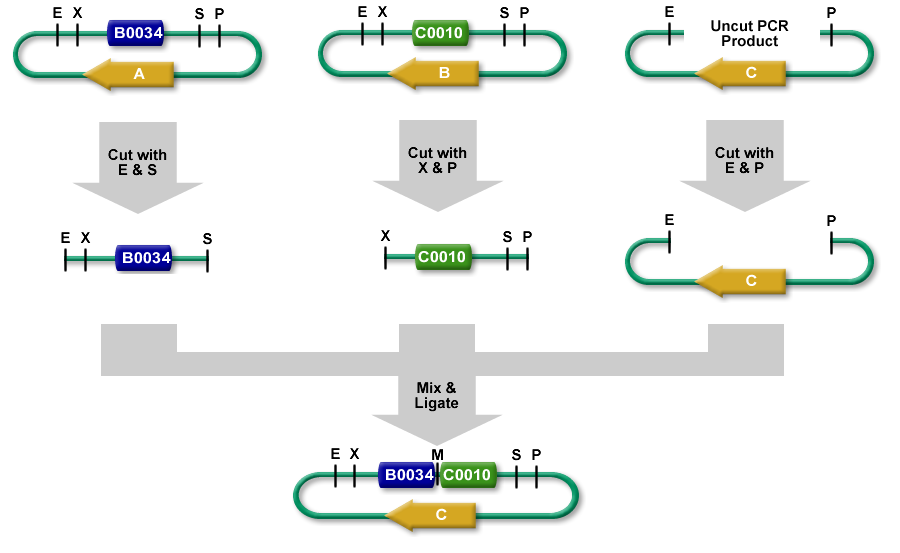
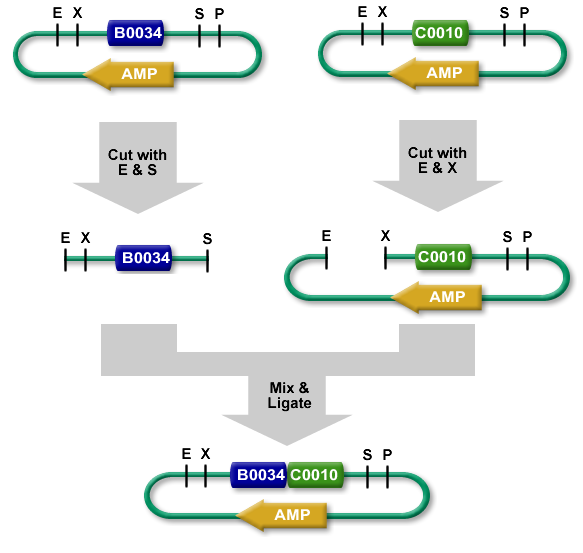
3A Assembly (which stands for three antibiotic assembly) is a method for assembling two part samples and selecting for correct assemblies through antibiotics. 3A assembly uses the restriction sites on the prefix and suffix to assemble part samples. This new composite part will maintain the same prefix and suffix as its "parents" and contain a scar, where the cut and re-ligated restriction sites were stitched together.
It uses effective antibiotic selection to eliminate unwanted background colonies and eliminates the need for gel purification and colony PCR of the resulting colonies. In theory, about 97% of the colonies should be the desired assembly. Additionally, the Registry has been providing iGEM teams and Registry labs with linearized plasmid backbones to further improve this assembly method.
3A Assembly Advantages
- No PCR
- No gel purification
- Higher success rate compared to Standard Assembly.
Links
Type IIS Assembly Methods
Type IIS restriction enzymes, like BsaI, cut DNA outside of their recognition site. This allows for custom overhangs, unlike normal restriction based enzymes (EcoRI cuts at its recognition site creating the same overhang every time). Type IIS assembly methods, such as MoClo, use this to their advantage. Parts will have the same restriction enzyme but distinct custom overhangs, so multiple parts can be assembled at once (one pot assembly).
Type IIs assembly requires careful planning and preparation to ensure the multiple parts will assemble properly and in the correct order. Assembly standards like MoClo, help make this process easier by standardizing part.
Currently, the Registry is evaluating MoClo, to support it as another assembly systems.
Scarless Assembly
There are assembly methods which also allow for the construction of composite parts without scars or specific linkers, which is particularly useful for the assembly of proteins. Additionally, these methods may be able to circumvent assembly standard incompatibility between part samples: a part sample in a Silver RFC[23] plasmid backbone can be assembled with a part sample in a Berkeley RFC[21] plasmid backbone.
DNA Synthesis
Just as DNA synthesis can be used to construct a new basic part, it may also be used to assemble parts together. Using the Registry database you can pull the sequence for parts you're interested, put them together in series, and then send the new composite sequence off to be synthesized.
See the DNA synthesis page for more information and our offer with IDT.
Gibson Assembly
Gibson Assembly has not been tested by the Registry yet, but several teams have had success with this assembly method. The [http://2010.igem.org/Team:Cambridge/Gibson/Introduction Cambridge 2010 iGEM Team] developed a set of protocols and tools that may be useful.
The Registry will be evaluating Gibson Assembly, and will have resources available for this assembly method available soon.
Discontinued Assembly Methods
Standard BioBrick Assembly
For Standard BioBrick Assembly, a part sample is cut out from its plasmid backbone and inserted into the prefix of a plasmid backbone of another part. Two restriction digests are done, one for the part sample that will be moved and one for the plasmid backbone that will receive it. The digests are then run on a gel and using gel purification the required fragments are isolated (the part sample and the cut plasmid backbone). The purified insert and cut plasmid backbone are ligated and the resulting composite part can be transformed into E.coli cells.
Due to its use of gel purification and lower success rate, the Registry no longer recommends the Standard BioBrick Assembly method.