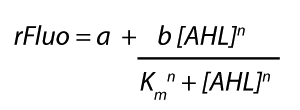Difference between revisions of "Part:BBa I14017:Experience"
(→Results) |
(→Background information) |
||
| Line 30: | Line 30: | ||
== Background information == | == Background information == | ||
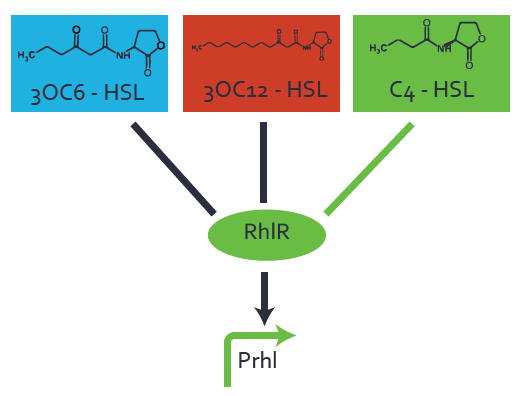
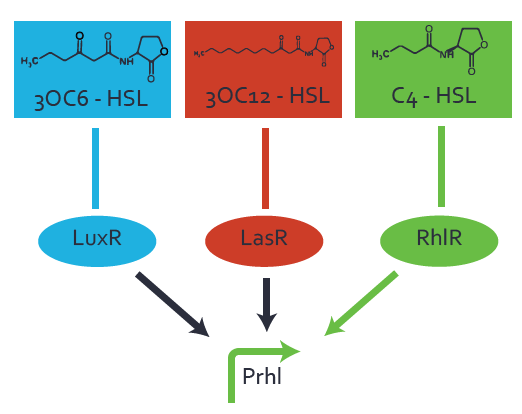
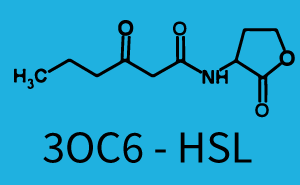
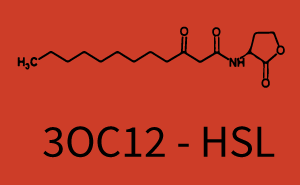
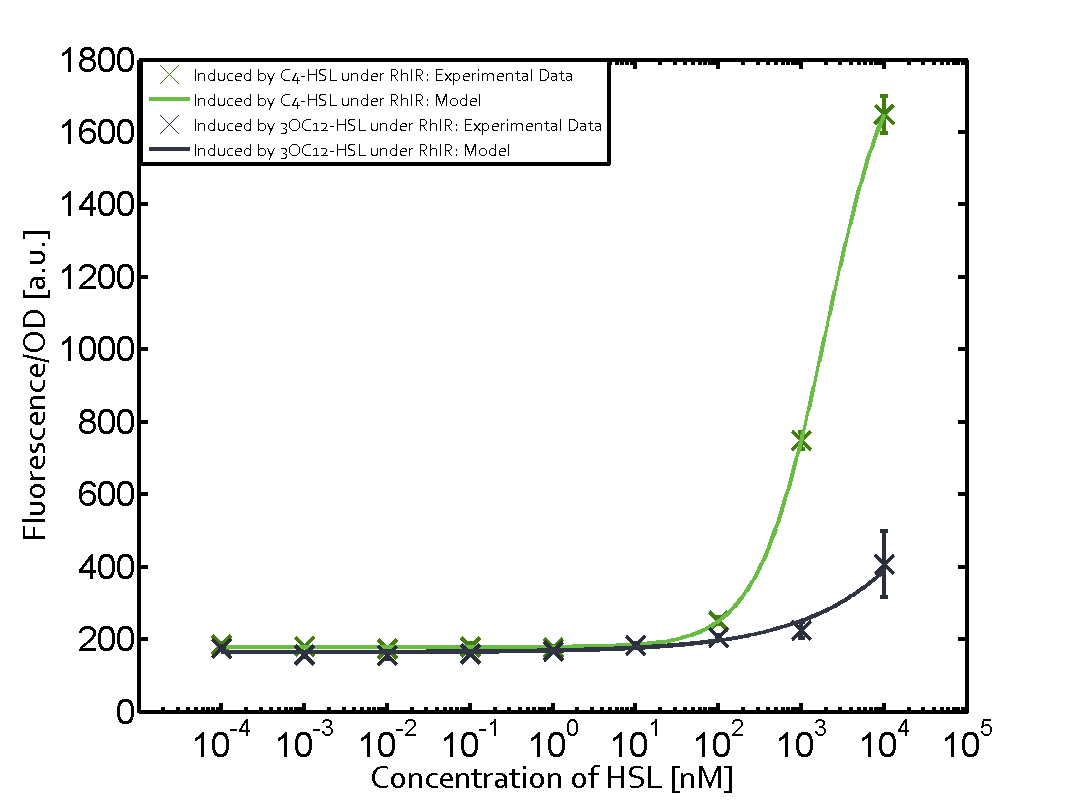
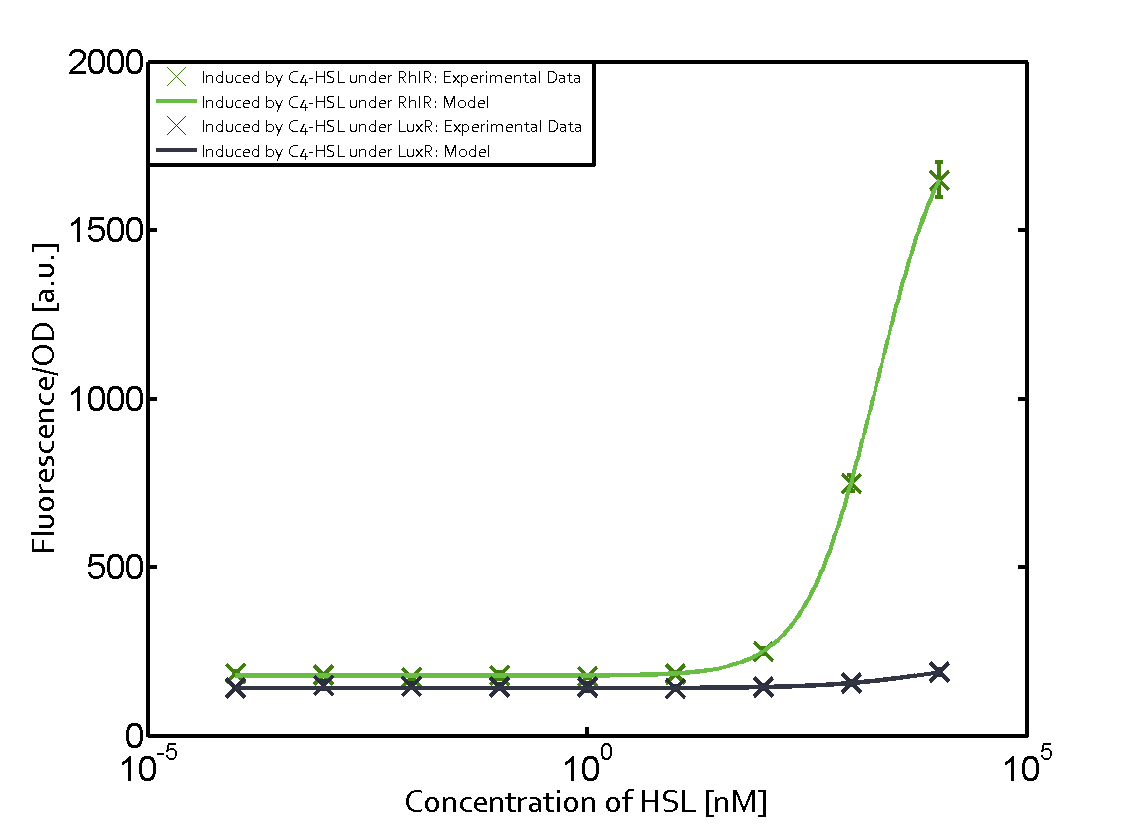
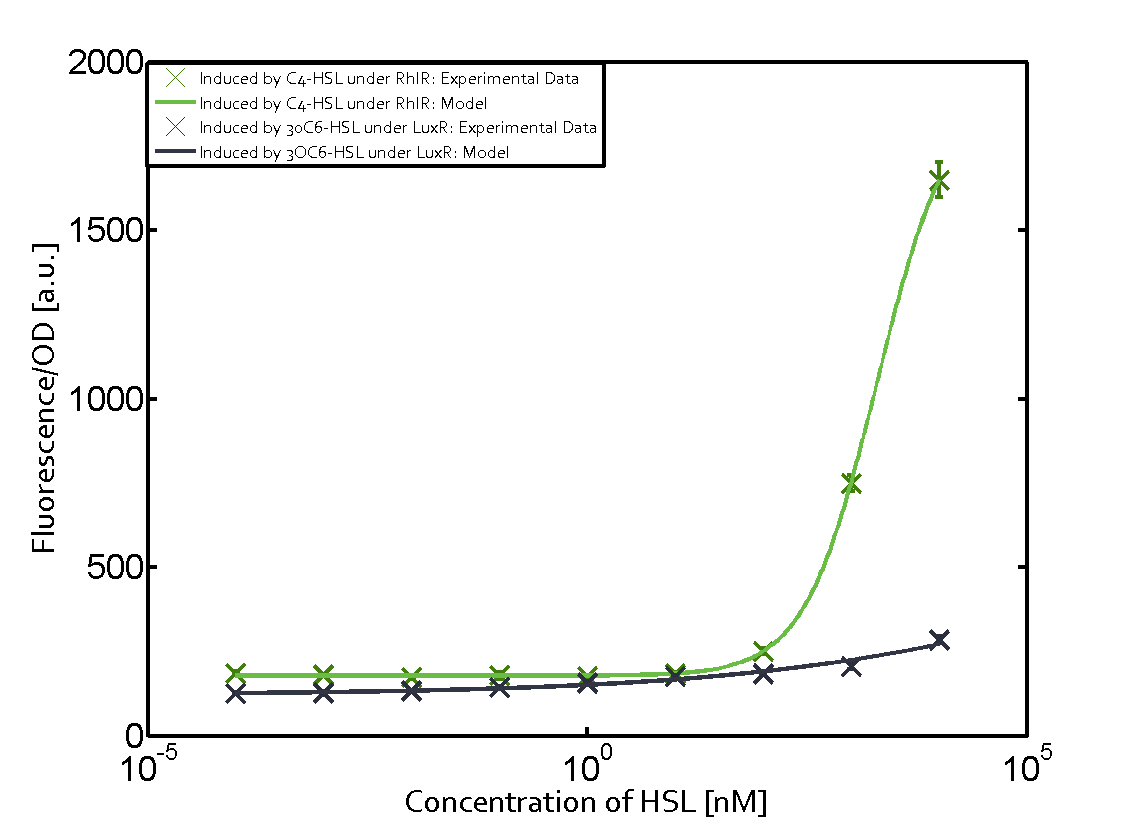
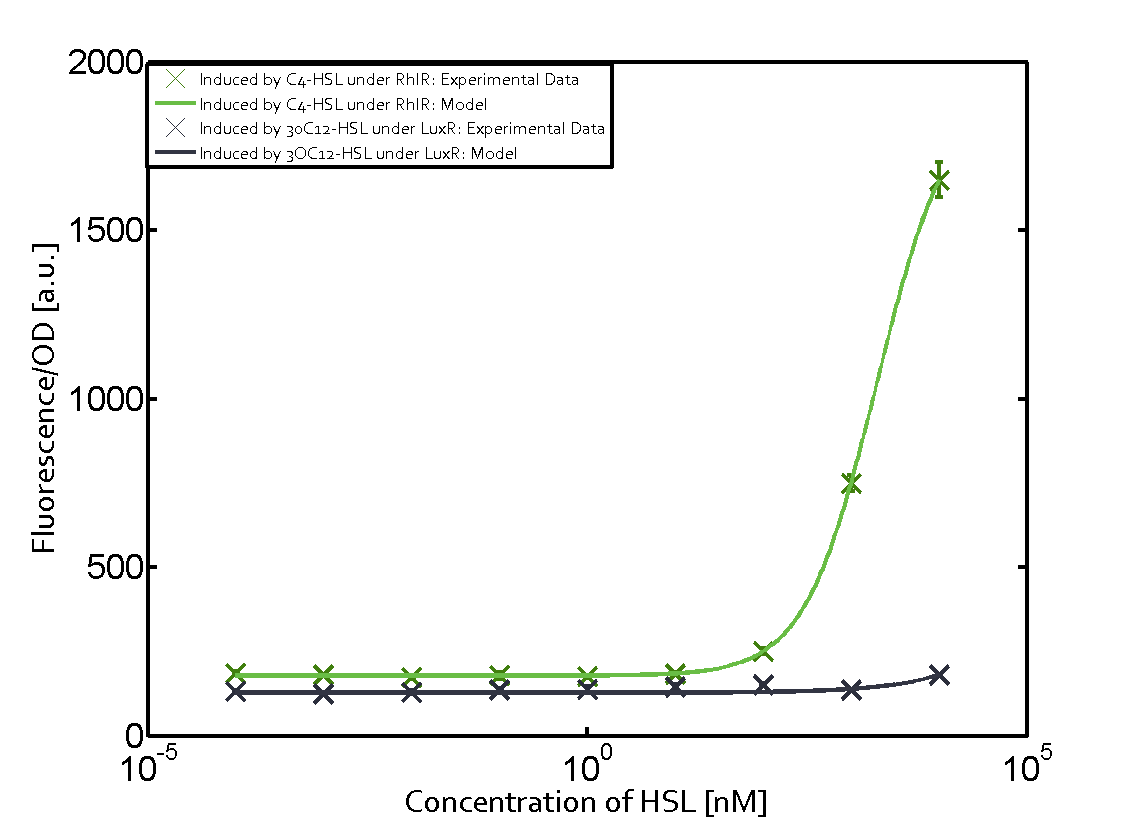
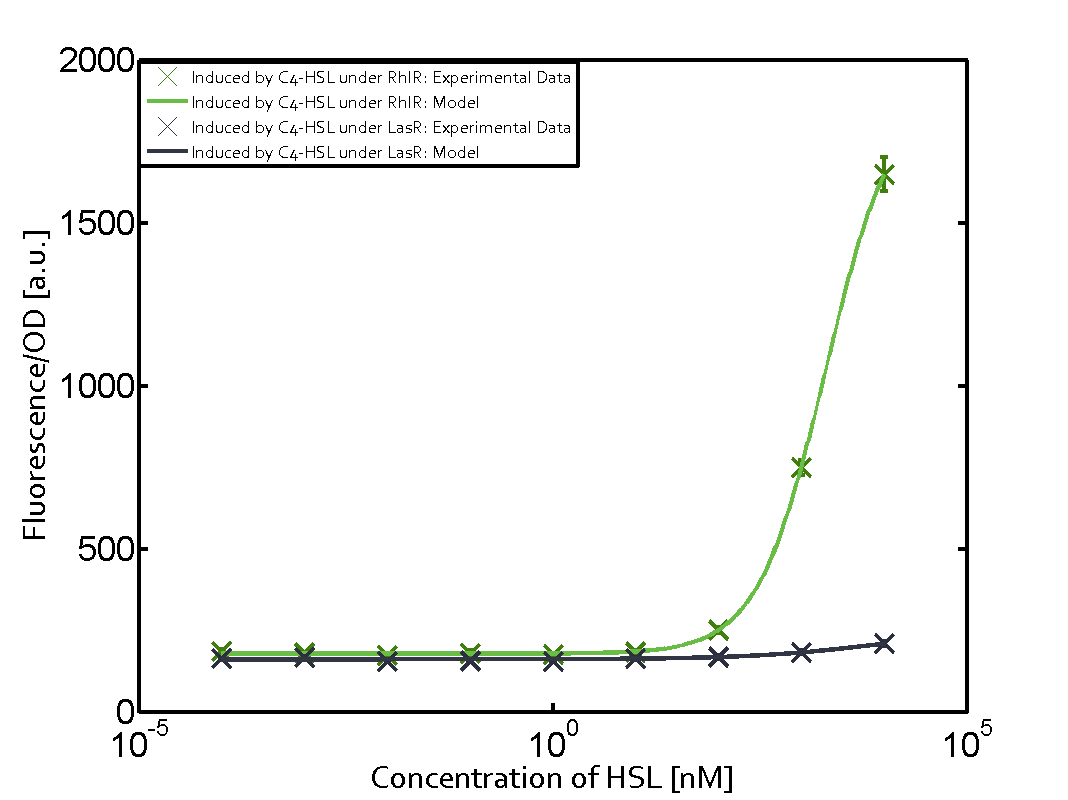
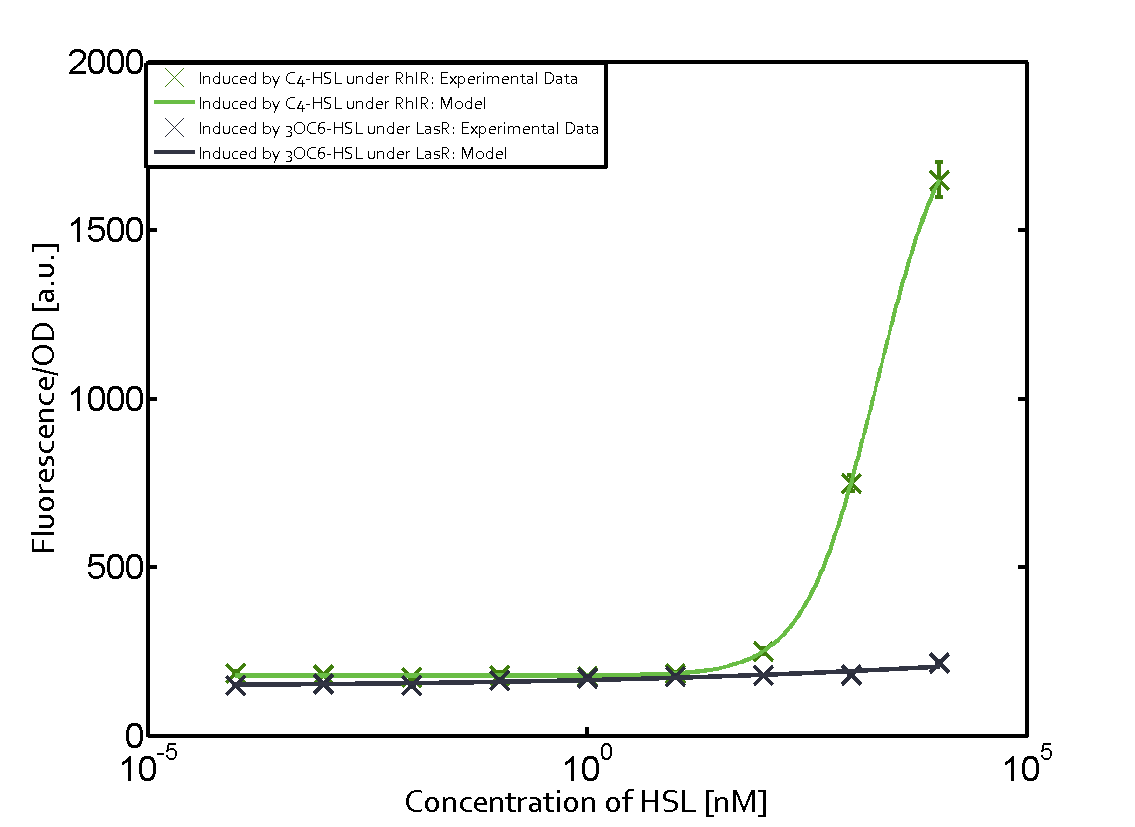
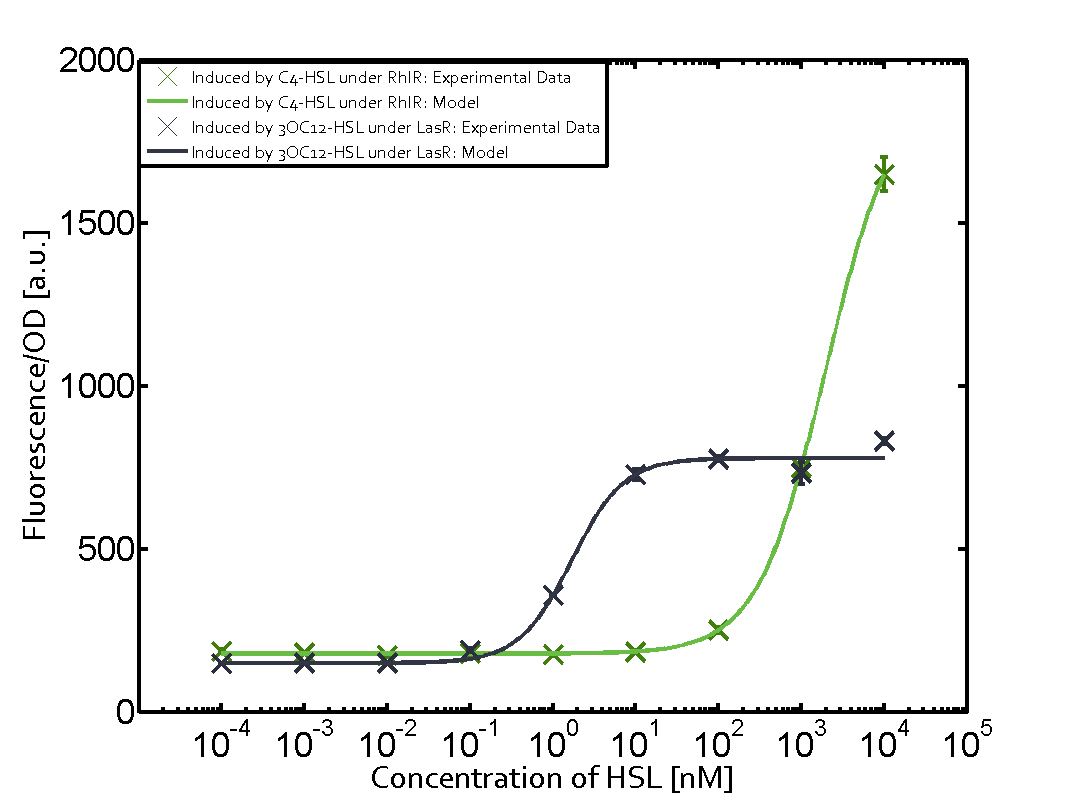
| − | + | The ''E. coli'' strain used and the experimental set-up are described above. However, here we focus on the characterization of crosstalk and as a result we used only one, strong promoter [https://parts.igem.org/Part:BBa_J23100 (BBa_J23100)] controlling the three different regulators ([https://parts.igem.org/Part:BBa_C0062 LuxR], [https://parts.igem.org/Part:BBa_C0179 LasR], and [https://parts.igem.org/Part:BBa_C0171 RhlR]) used in the experiments in order to quantify crosstalk with [https://parts.igem.org/Part:BBa_I14017 pRhl]. In the following, we describe all the different levels of crosstalk we have assessed. | |
== First-order crosstalk == | == First-order crosstalk == | ||
Revision as of 14:18, 24 October 2014
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_I14017
User Reviews
UNIQ2ae0f423aa0f0d38-partinfo-00000000-QINU
|
••••
ETH Zurich 2014 |
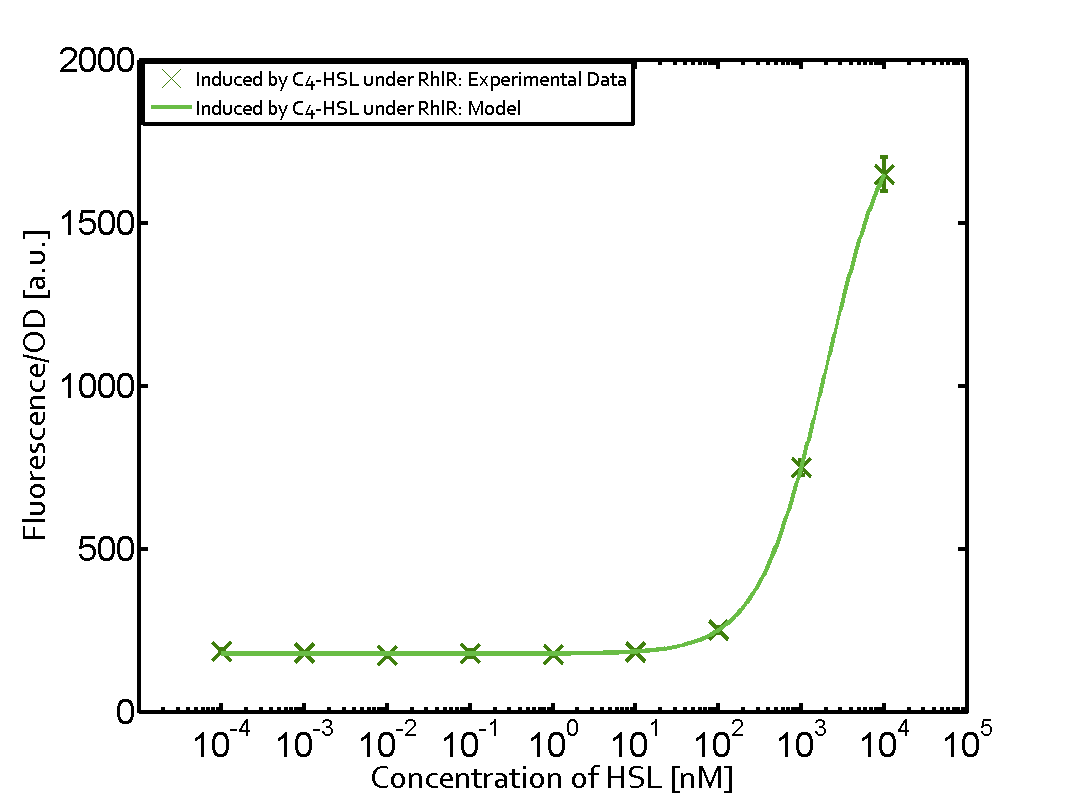
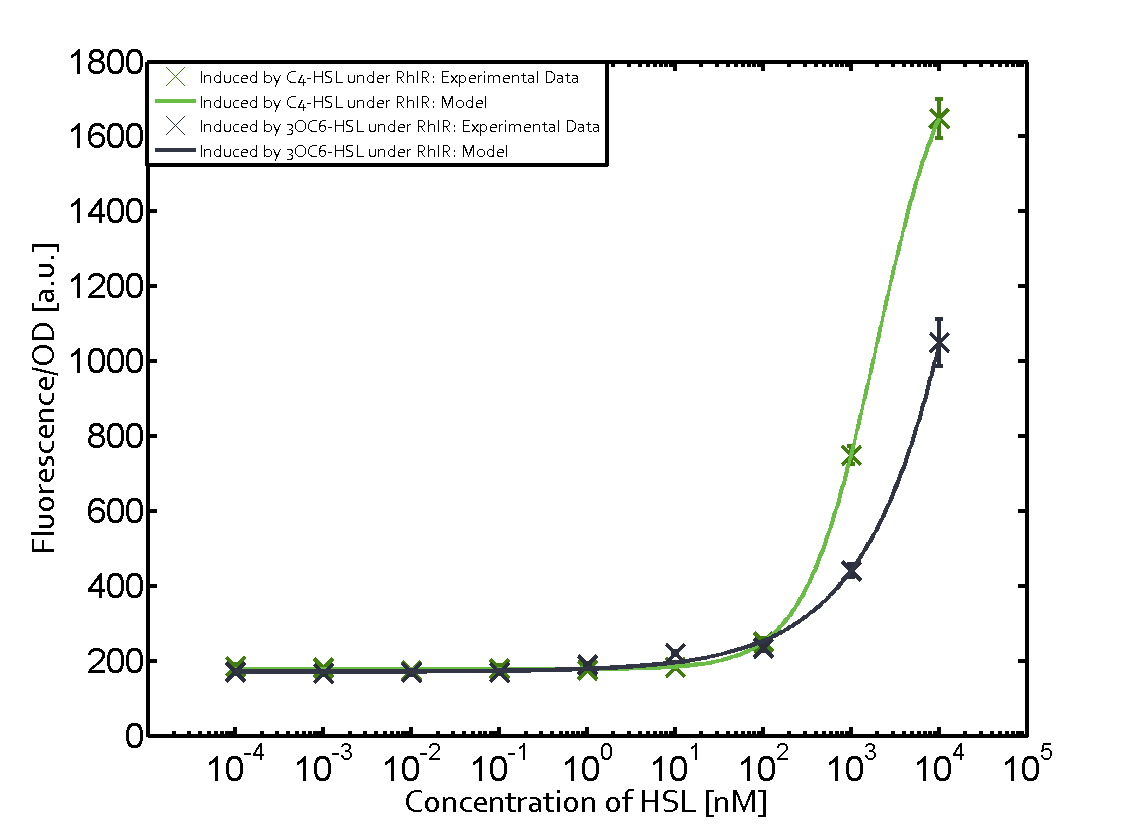
Characterization of two-order crosstalk on the promoterBackground informationThe E. coli strain used and the experimental set-up are described above. However, here we focus on the characterization of crosstalk and as a result we used only one, strong promoter (BBa_J23100) controlling the three different regulators (LuxR, LasR, and RhlR) used in the experiments in order to quantify crosstalk with pRhl. In the following, we describe all the different levels of crosstalk we have assessed. First-order crosstalkFirst Level crosstalk: RhlR binds to different HSL and activates the promoterSecond Level crosstalk: other regulatory proteins, like LuxR, bind to their natural HSL substrate and activates the promoterSecond order crosstalk: Combination of both cross-talk levelsOther regulatory proteins, like LuxR, bind to different HSL and activates the promoter. Results
Modeling crosstalkEach experimental data set was fitted to an Hill function using the Least Absolute Residual method. The fitting of the graphs was performed using the following equation :
| ||||||||||||||||||||||||||||||||||||
|
Antiquity |
This review comes from the old result system and indicates that this part did not work in some test. |
UNIQ2ae0f423aa0f0d38-partinfo-00000003-QINU