Part:BBa_K3457003
T7 promoter
T7 promoter of pet-modification vector
Contribution
Group: QHFZ-China iGEM 2020
This part is a basic part. QHFZ-China 2020 measured it in their composite parts, K3457032, K3457033, K3457034, K3457035, K3457036, K3457037, K3457039, K3457042, K3457043.
Contribution from iBowu-China 2021
In iGEM 2021, Team iBowu-China adopted T7 promoter to use in combination with LacO and gusA sequence from E. coli and other sources to express an enzyme β-glucuronidase. In our design, we tested the use of this "short" T7 promoter and noted the difference with a complete T7 promoter [1].
In Figure 2, the first promoter sequence is short T7 promoter part on this page, while the second T7 promoter is in full length with four more bases, documented on the respective pages.
Introduction
T7 promoter is a commonly used promoter, which can be recognized by T7 RNA polymerase and efficiently transcribe downstream genes. This year we used this promoter to express sfGFP or β-glucuronidase enzyme in strains such as E. coli BL21 (DE3) to express green fluorescence, or to convert glycyrrhizic acid into glycyrrhetinic acid. We used the T7 promoter part K3457003 established and measured by QHFZ-China 2020, but we found that this element cannot initiate downstream gene expression. Further research found that after adding four bases of GGAA to it, the function returned to normal. In fact we found that pet28a, a commonly used expression vector, has two connected features on its sequence, the T7 promoter and the LacO sequence, and these two sequences actually overlap. Specifically, the overlap is the four GGAAs at the end of the T7 promoter. The bases are also included in the LacO feature. We guess that QHFZ-China 2020 overlooked this problem when splitting these two elements, resulting in the lack of four necessary bases in the T7 promoter designed. In addition, according to the literature [1], we have designed three other promoters, C4, H9 and G6. They can also be activated by T7 RNA polymerase, but the strength is different from that of T7 promoter, so that users can choose the right promoter according to the required expression strength.
Protocol
- Transform the plasmids into E. coli BL21(DE3)
- Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 4ml LB medium with kanamycin. Add 1mM IPTG to all experimental groups as needed. Incubate at 37℃ in a shaker overnight.
- Fluorescence. Acquire 100 ul bacteria culture and centrifuge at 10000g for 1 min. Aspirate the supernatant and resuspend with 100 ul ddH2O to get rid of the fluorescence background from LB medium. Add 100 µl sample solution into a sterile 96-well plate. Measure fluorescence with a microplate reader and then also measure OD600 for normalization.
- SDS-PAGE.
- Take 1ml of bacterial solution, centrifuge at 12000g for 1min at 4℃, discard the supernatant, add 100ul of pre-cooled RIPA lysate (high), vortex to mix, settle for 5min, centrifuge at 4℃, 12000g for 10min, and take the supernatant which contains the protein extract. After protein quantification by BCA method using commercial test box, adjust the concentration of the protein solution to 1mg/ml. Take 4 parts of the protein solution and 1 part of 5X protein loading buffer and mix it in a boiling water bath for 5 minutes, centrifuge at 12000g at 4°C for 10 minutes, take the supernatant, and store on ice. Use precast gel purchased from commercial company (Transgen, Precast Tris-Glycine Gel) for protein electrophoresis.
- Add marker in the first lane on the left at 5μl, and add sample on all other lanes with 10ul sample on each lane. Use 100V for electrophoresis. After the electrophoresis, take out the gel, put it in the staining box, add Coomassie Brilliant Blue dye solution to about 3mm below the gel surface, shake on a horizontal shaker, dye for 30min at room temperature, discard the dye solution, wash off the floating color with distilled water, and add decolorization liquid, decolorize on a horizontal shaker until the result is in good condition for taking pictures.
- Enzyme Activity
- Take 100 ul of bacterial solution, centrifuge at 10000g for 1min at room temperature and discard the supernatant. Resuspend with 100ul ddH2O, and add into this solution 100ul of standard test solution I (containing Phenolphthalein-β-D-glucuronide, and the test box was purchased from Nanjing Jiancheng Biotech company). Incubate the mixture at 37C for 1 hour.
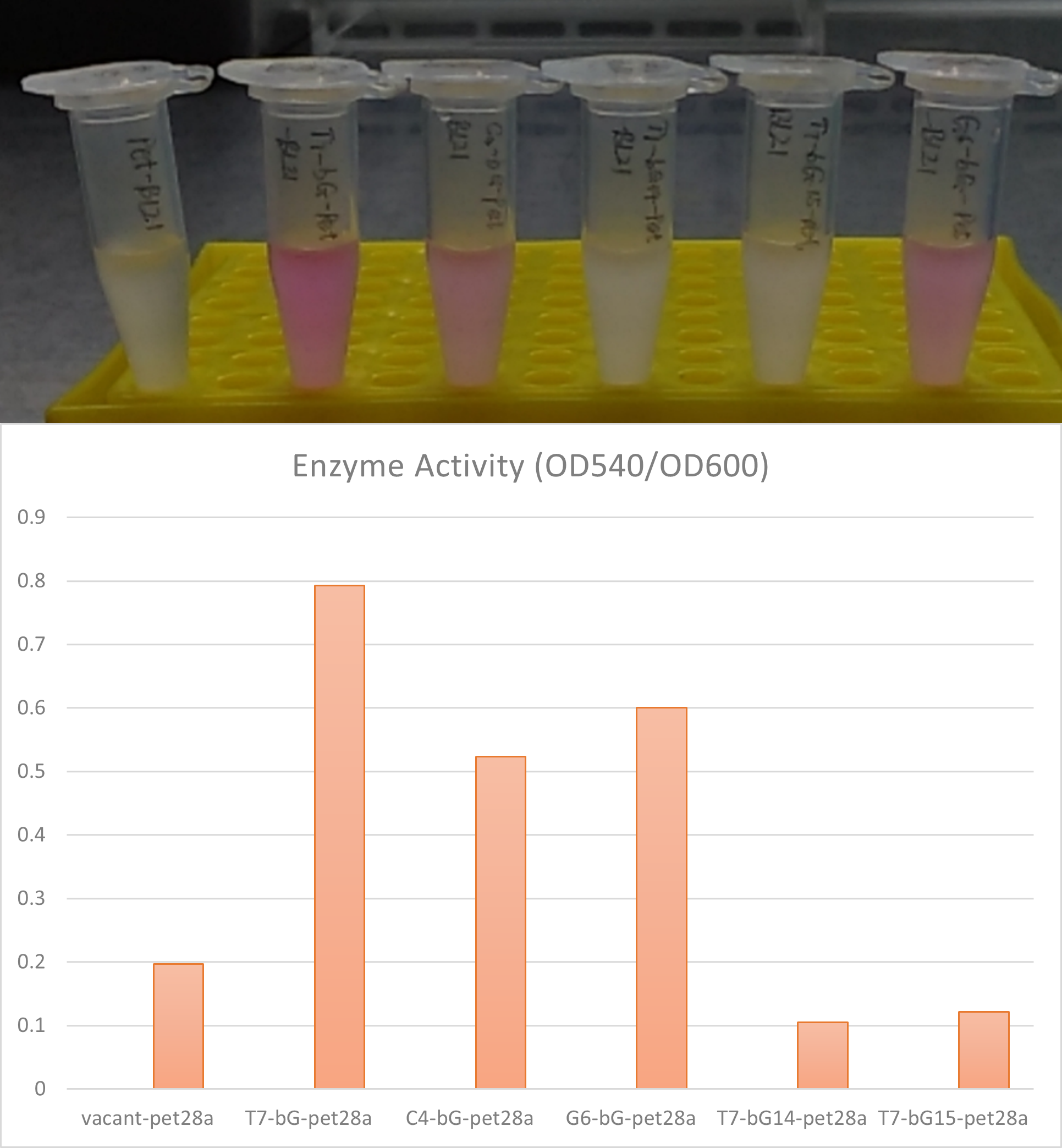
- Observe the solution. pink or purple color indicates positive enzyme activity. Quantitatively the activity can be measured by OD540 reading on a microplate reader, divided by its OD600 reading for normalization of concentration.
Results
We added the above-mentioned promoters to the upstream of the sfGFP gene, and determine the intensity of each promoter by measuring its green fluorescence.
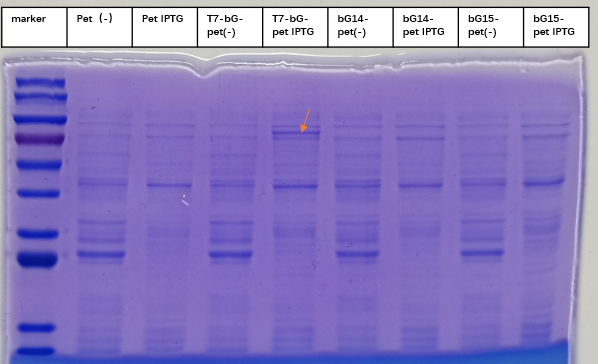
We also put the T7 promoter with GGAA in the upstream of the β-glucuronidase enzyme. Through SDS-PAGE, it can be found that this composite part can efficiently initiate the expression of the β-glucuronidase enzyme.
Further, the β-glucuronidase enzyme has been proved to show enzyme activity.
References
[1] Jones, J., Vernacchio, V., Lachance, D. et al. ePathOptimize: A Combinatorial Approach for Transcriptional Balancing of Metabolic Pathways. Sci Rep 5, 11301 (2015). https://doi.org/10.1038/srep11301
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |