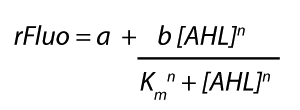Difference between revisions of "Part:BBa C0062:Experience"
(→Characterization of the promoter's sensitivity to 3OC6-HSL depending on LuxR concentration) |
(→Results) |
||
| Line 32: | Line 32: | ||
== Results == | == Results == | ||
| − | [[File:ETH_Zurich_2014_Different_LuxRConc.png|500px|thumb|center|'''Figure 1''']] | + | The measurements of the induced system with [[3OC6HSL|3OC6-HSL]] concentrations of 10<sup>-13</sup> M to 10<sup>-5</sup> M showed an increasing sensitivity of the [https://parts.igem.org/Part:BBa_R0062 pLux (BBa_R0062)] promoter (in terms of fluorescence per OD<sub>600</sub>) for increasing strength of the promoter controlling [https://parts.igem.org/Part:BBa_C0062 LuxR (BBa_C0062)] expression. For [https://parts.igem.org/Part:BBa_J23100 BBa_J23100] (strongest promoter chosen) the sensitivity is highest (half maximal effective concentration EC<sub>50</sub> approximately 20 pM), for [https://parts.igem.org/Part:BBa_J23109 BBa_J23109] (weakest one chosen) the sensitivity is lowest (EC<sub>50</sub> approximately 100 pM), with [https://parts.igem.org/Part:BBa_J23111 BBa_J23111] (medium) falling between these two but closer to the strong promoter (EC<sub>50</sub> approximately 10 nM). Overall, this is in line with the promoter strength given in the [https://parts.igem.org/Promoters/Catalog/Anderson Anderson collection] and suggests that the sensitivity of the [https://parts.igem.org/Part:BBa_R0062 pLux (BBa_R0062)] promoter increases with an increase of [https://parts.igem.org/Part:BBa_C0062 LuxR (BBa_C0062)] expression. Also, the sensitivity and the approximate EC<sub>50</sub> values are in the range of previous experience. |
| + | |||
| + | |||
| + | [[File:ETH_Zurich_2014_Different_LuxRConc.png|500px|thumb|center|'''Figure 1 Effect of varied promoter strength for the expression of [https://parts.igem.org/Part:BBa_C0062 LuxR (BBa_C0062)] and the resulting change of [https://parts.igem.org/Part:BBa_R0062 pLux (BBa_R0062)] sensitivity.''' The fluorescence per OD<sub>600</sub> is shown over an inducer-range of 10<sup>-13</sup> M to 10<sup>-5</sup> M. The promoters used are: [https://parts.igem.org/Part:BBa_J23100 BBa_J23100] (high LuxR expression, red), [https://parts.igem.org/Part:BBa_J23111 BBa_J23111] (medium LuxR expression, green), and [https://parts.igem.org/Part:BBa_J23109 BBa_J23109] (low LuxR expression, blue). All three promoters are part of the [https://parts.igem.org/Promoters/Catalog/Anderson Anderson collection]. Data points are mean values of triplicate measurements in 96-well microtiter plates 200 min after induction ± standard deviation. For the full data set and kinetics please [http://2014.igem.org/Team:ETH_Zurich/contact contact] us or visit the [http://2014.igem.org/Team:ETH_Zurich/data/raw raw data] page.]] | ||
= Characterization of two-order crosstalk = | = Characterization of two-order crosstalk = | ||
Revision as of 20:58, 27 October 2014
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_C0062
User Reviews
UNIQ633255bcde4fd56d-partinfo-00000000-QINU
|
••••
ETH Zurich 2014 |
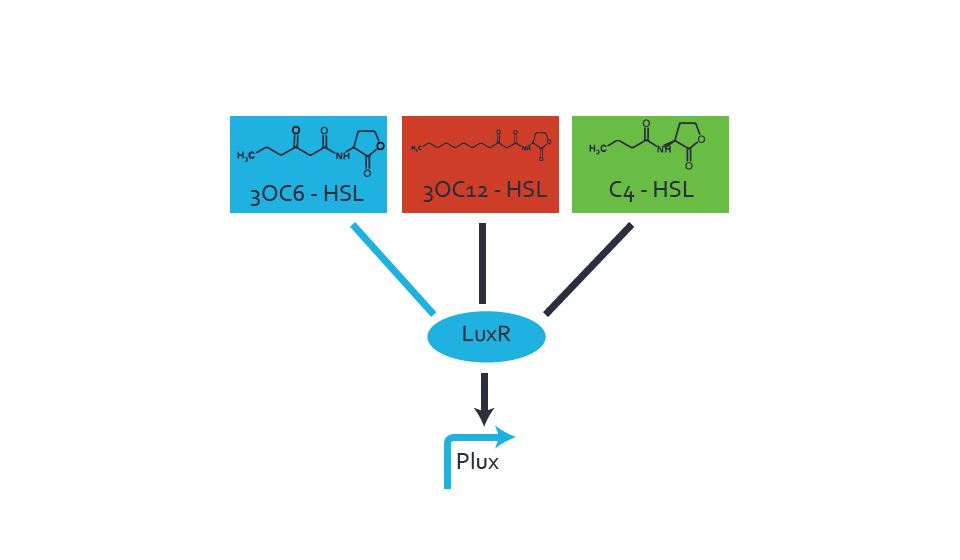
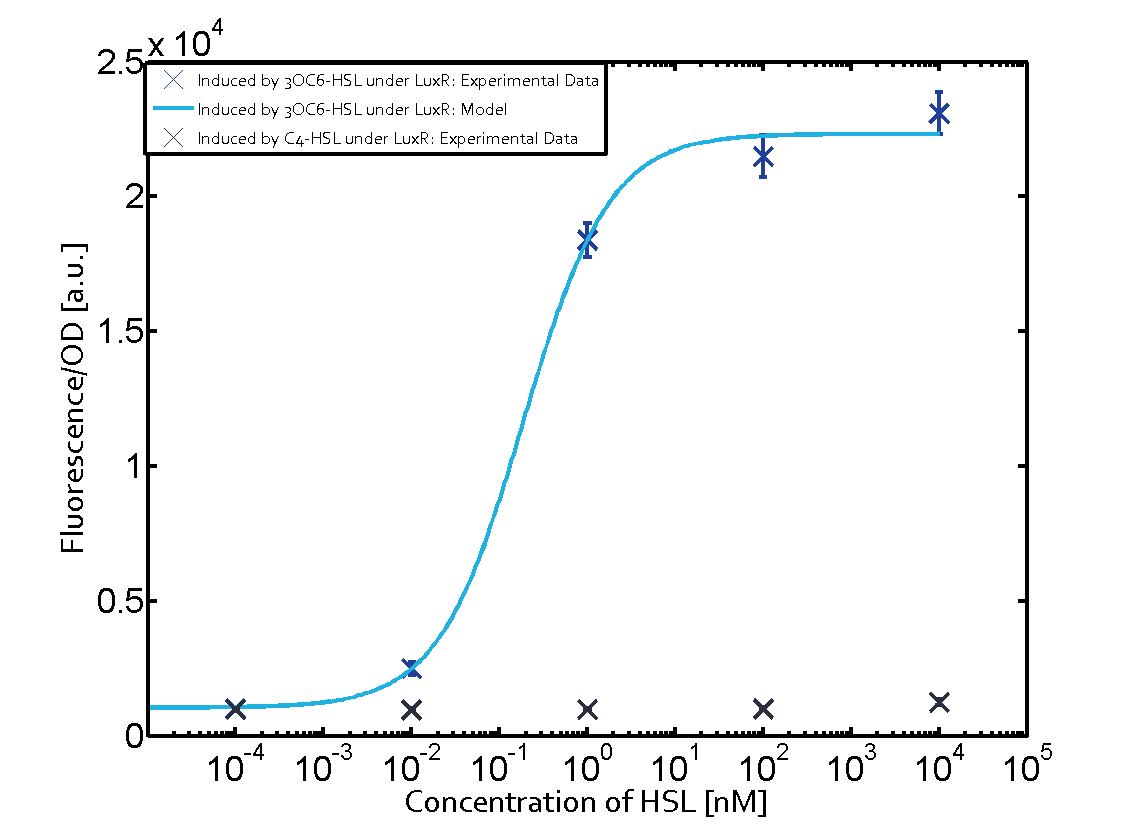
Characterization of the promoter's sensitivity to 3OC6-HSL depending on LuxR concentrationThe amount of regulator LuxR (BBa_C0062) in the system was shown to influence the pLux promoter's response to the inducer concentration (3OC6-HSL). By using the three different constitutive promoters BBa_J23100, BBa_J23109, and BBa_J23111 for the production of LuxR we have measured this effect in terms of fluorescence (see Figure 1). Background informationResultsThe measurements of the induced system with 3OC6-HSL concentrations of 10-13 M to 10-5 M showed an increasing sensitivity of the pLux (BBa_R0062) promoter (in terms of fluorescence per OD600) for increasing strength of the promoter controlling LuxR (BBa_C0062) expression. For BBa_J23100 (strongest promoter chosen) the sensitivity is highest (half maximal effective concentration EC50 approximately 20 pM), for BBa_J23109 (weakest one chosen) the sensitivity is lowest (EC50 approximately 100 pM), with BBa_J23111 (medium) falling between these two but closer to the strong promoter (EC50 approximately 10 nM). Overall, this is in line with the promoter strength given in the Anderson collection and suggests that the sensitivity of the pLux (BBa_R0062) promoter increases with an increase of LuxR (BBa_C0062) expression. Also, the sensitivity and the approximate EC50 values are in the range of previous experience.
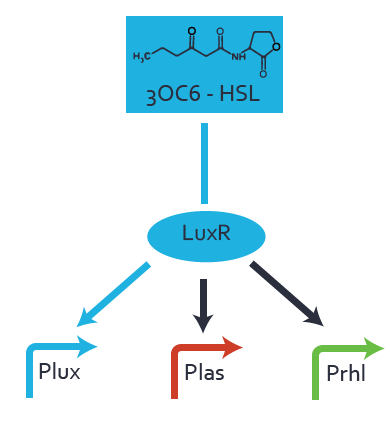
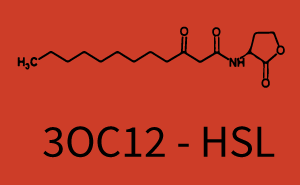
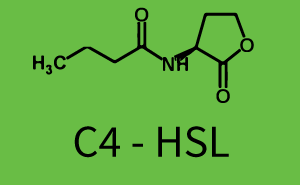
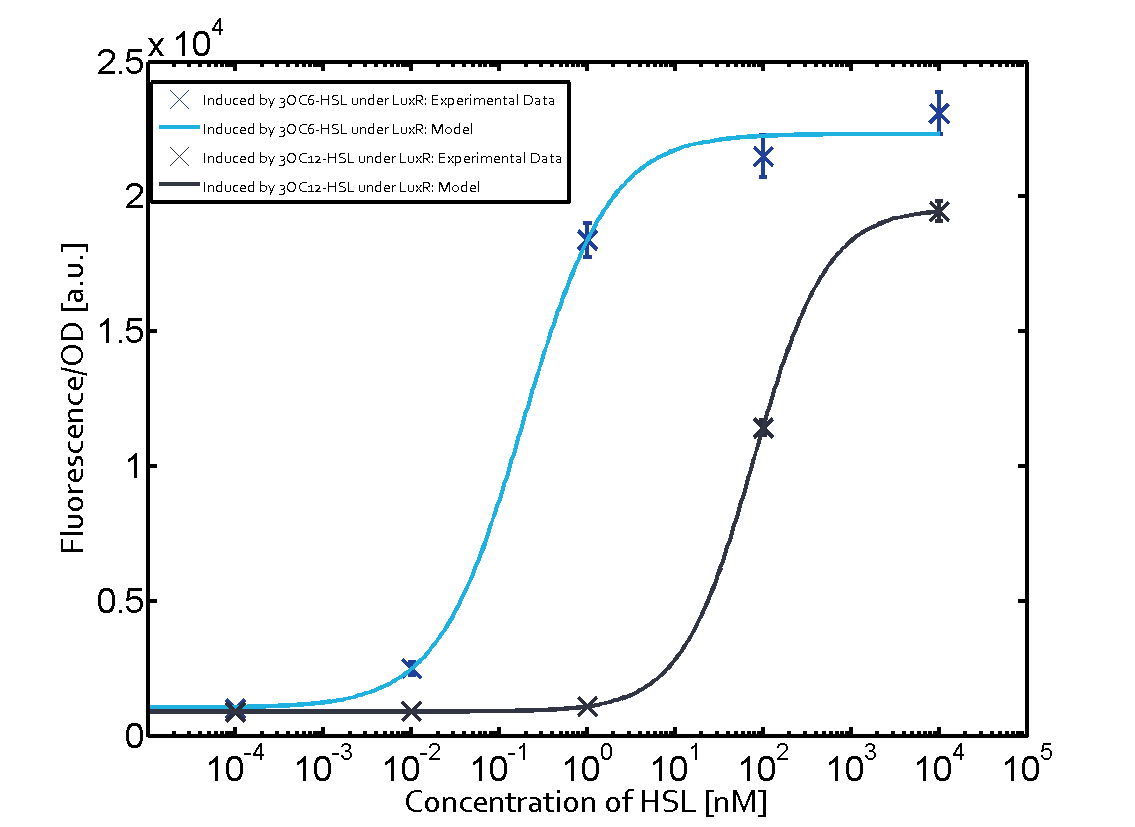
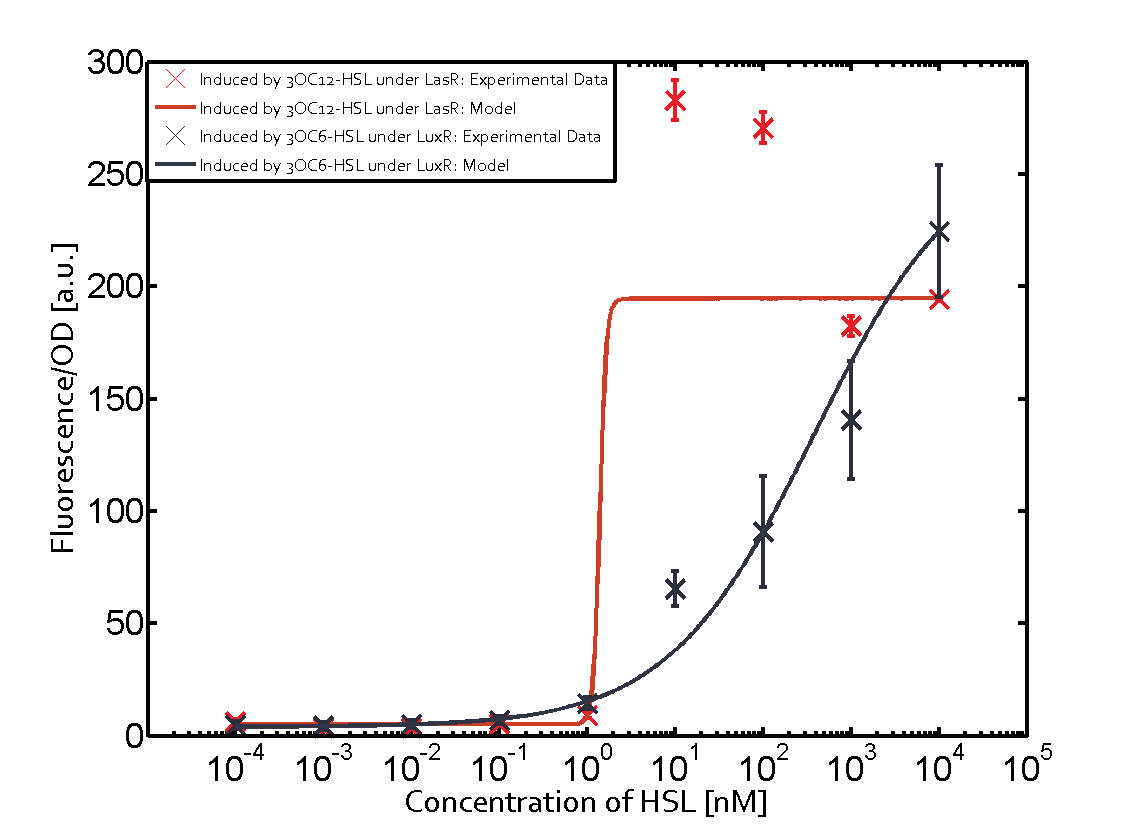
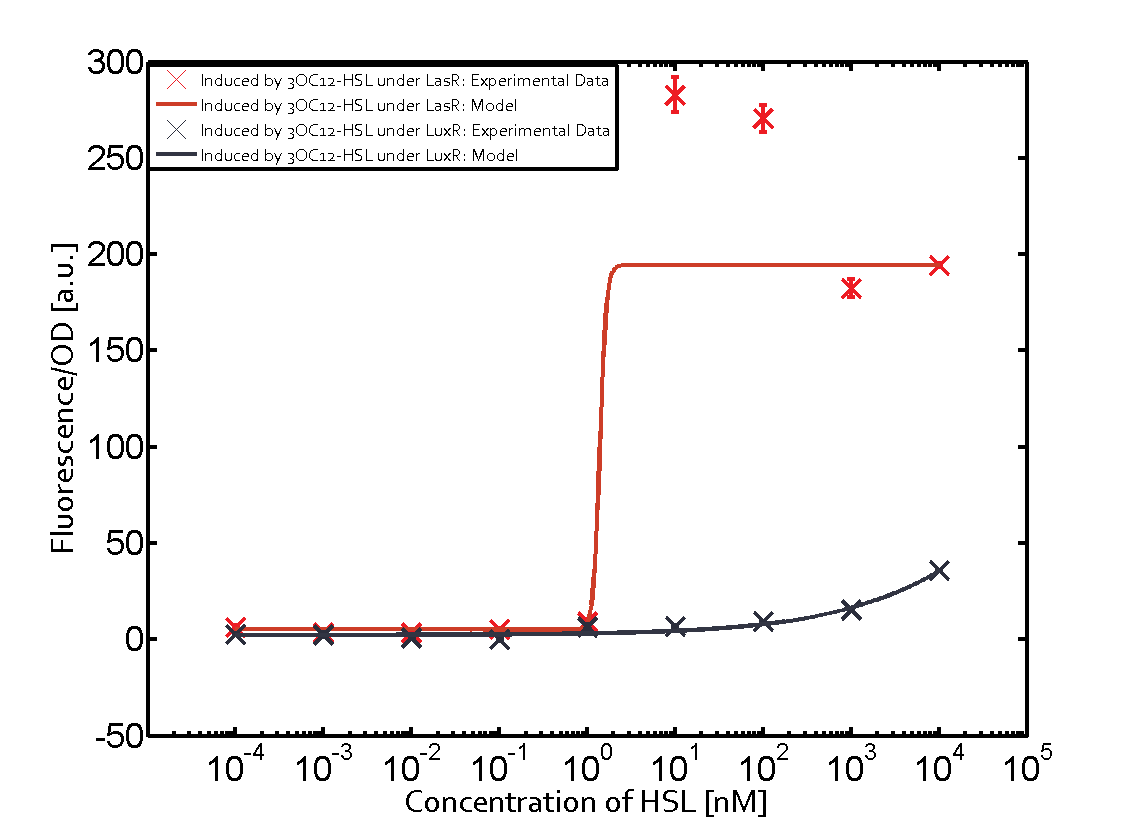
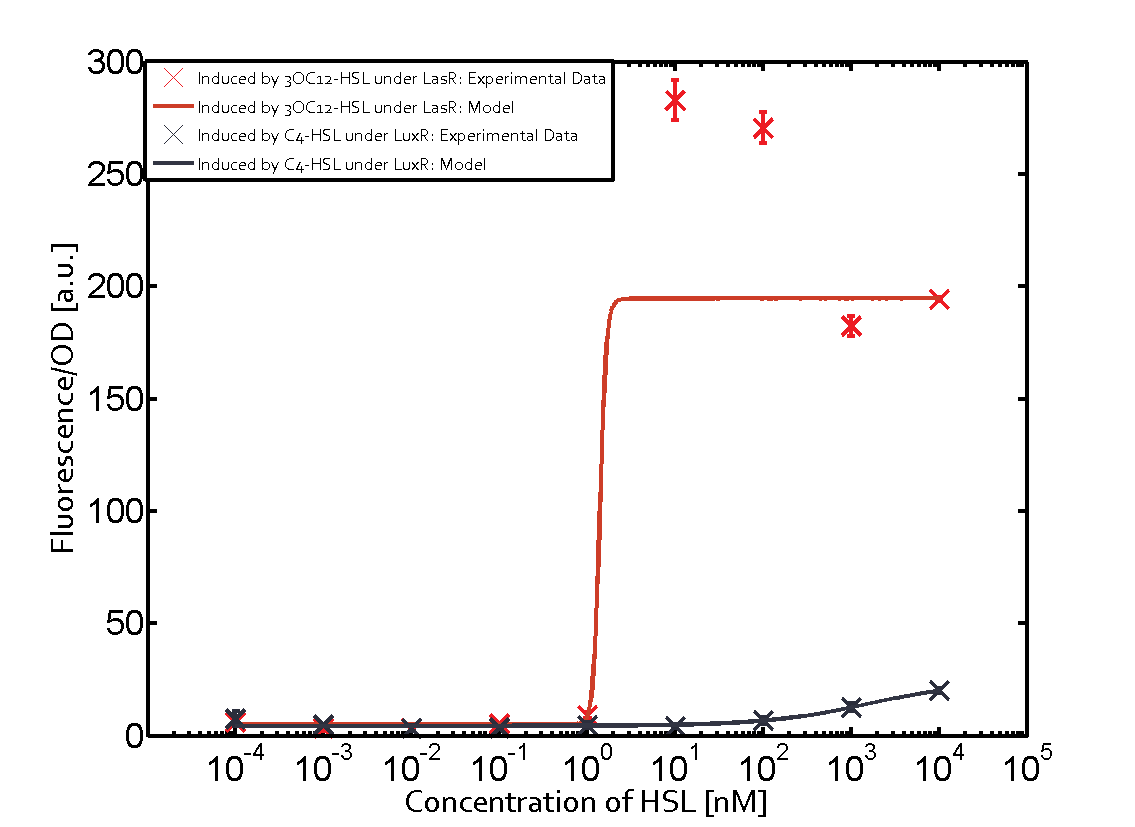
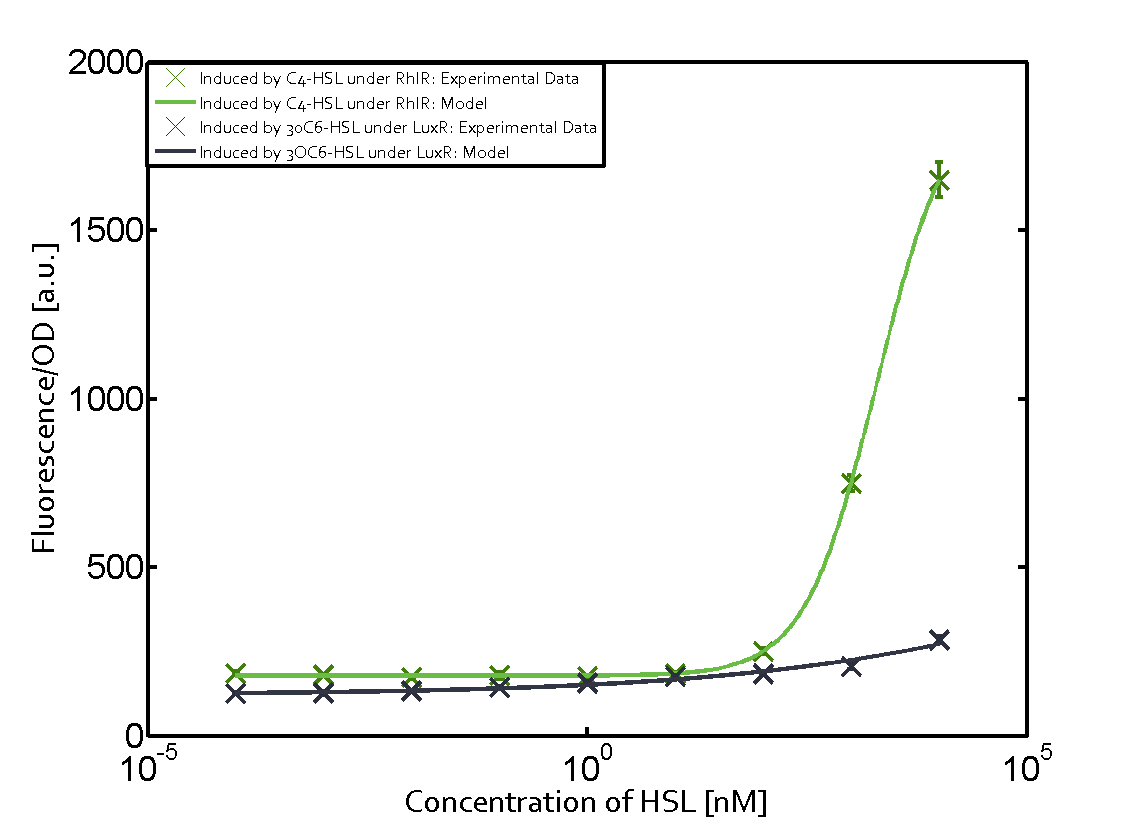
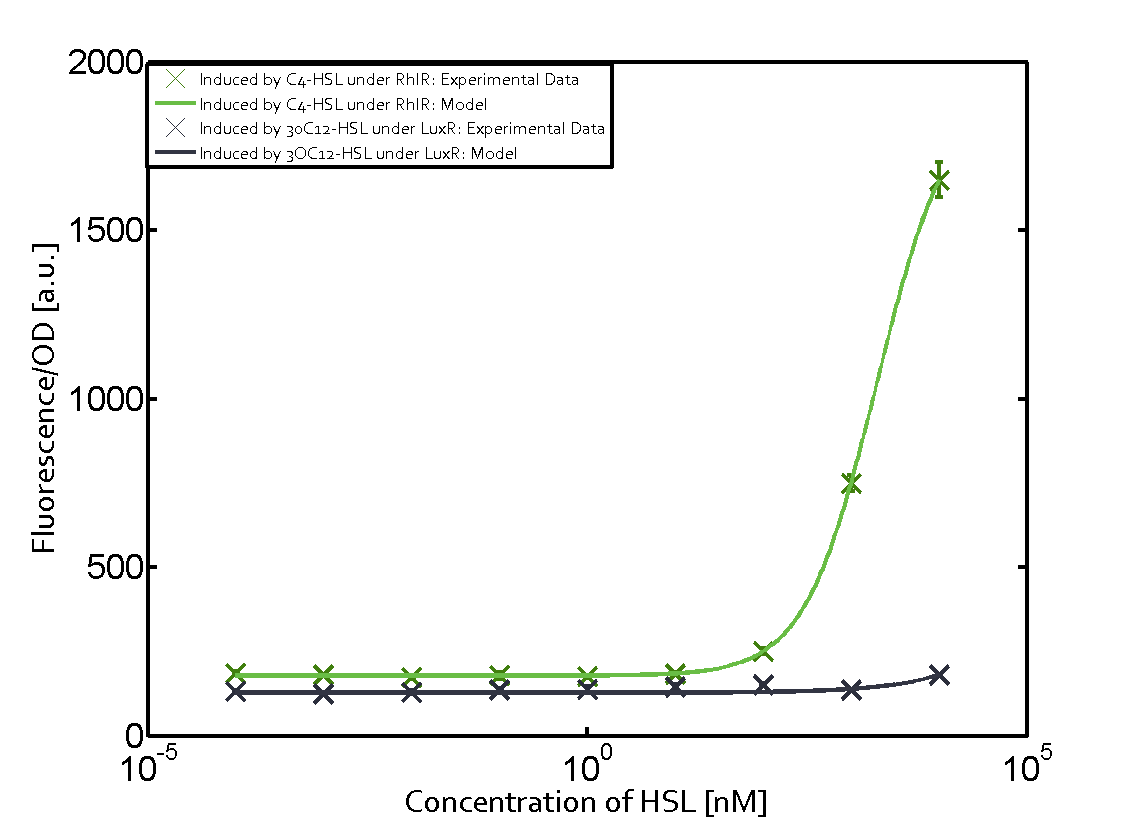
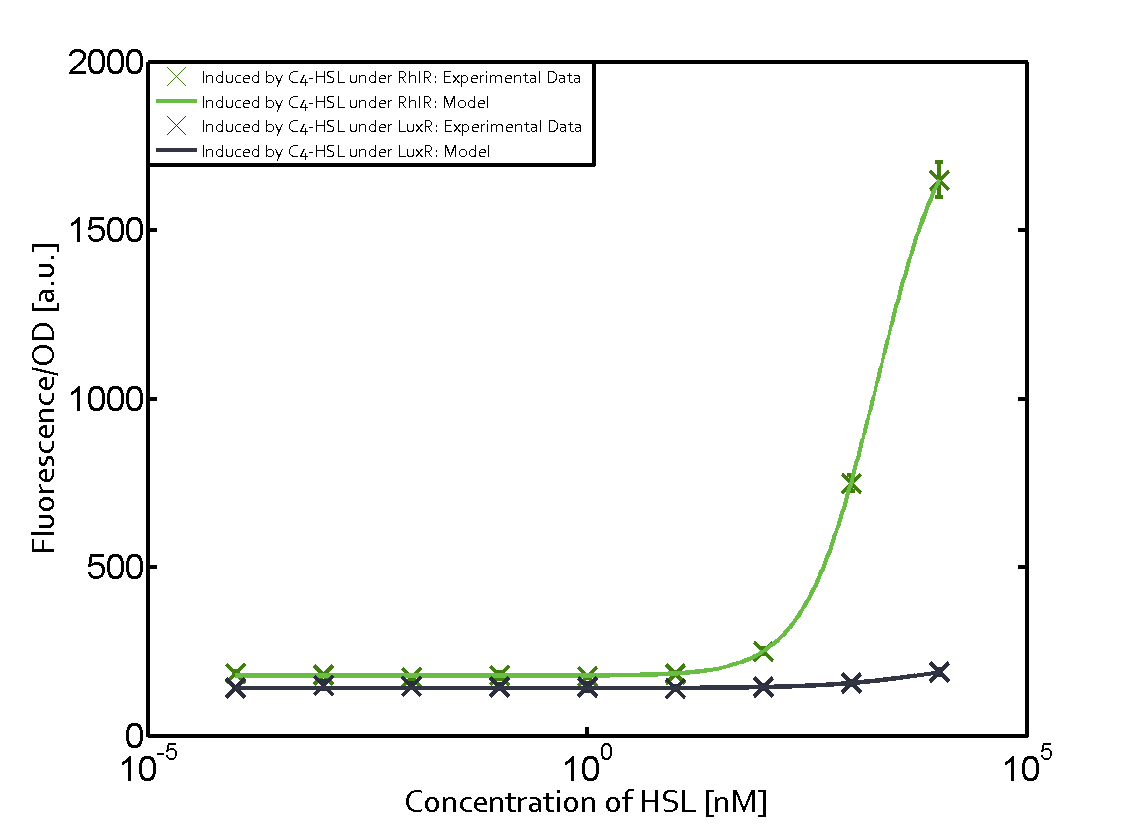
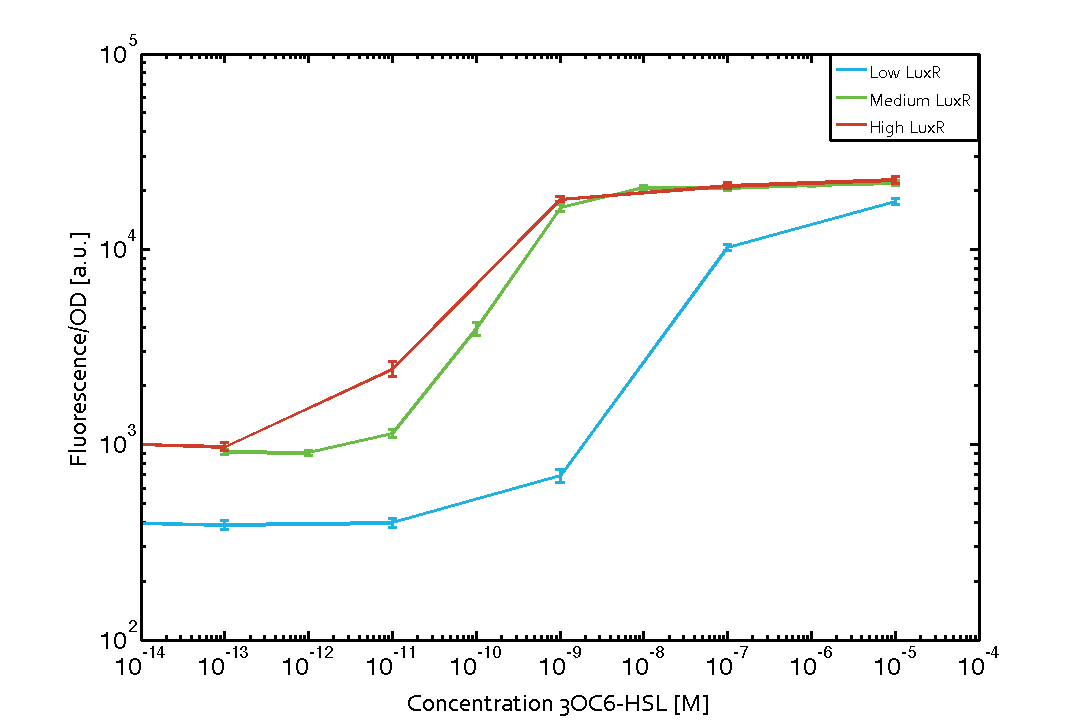 Figure 1 Effect of varied promoter strength for the expression of LuxR (BBa_C0062) and the resulting change of pLux (BBa_R0062) sensitivity. The fluorescence per OD600 is shown over an inducer-range of 10-13 M to 10-5 M. The promoters used are: BBa_J23100 (high LuxR expression, red), BBa_J23111 (medium LuxR expression, green), and BBa_J23109 (low LuxR expression, blue). All three promoters are part of the Anderson collection. Data points are mean values of triplicate measurements in 96-well microtiter plates 200 min after induction ± standard deviation. For the full data set and kinetics please [http://2014.igem.org/Team:ETH_Zurich/contact contact] us or visit the [http://2014.igem.org/Team:ETH_Zurich/data/raw raw data] page. Characterization of two-order crosstalkBackground informationHere, we focus on the characterization of crosstalk of LuxR with different AHLs and further crosstalk of LuxR-3OC6-HSL with the three promoters - pLux, pLas, and pRhl. In the following, we describe all the different levels of crosstalk we have assessed. First-order crosstalkIn the first order crosstalk section we describe activation of pLux due to LuxR binding to inducers different from 3OC6-HSL or pLux itself binding a regulator-inducer pair different from LuxR-3OC6-HSL. First Level crosstalk: LuxR binds to different HSL and activates the promoter PluxIn the conventional system 3OC6-HSL binds to its corresponding regulator, LuxR, and activates the pLux promoter (figure 2, light blue). However, LuxR can potentially also bind to other AHLs and then activate pLux (figure 2, 3OC12-HSL in red and C4-HSL in green). Second Level crosstalk: LuxR binds to 3OC6-HSL, its natural HSL, and activates different promotersSecond order crosstalk: Combination of both cross-talk levelsLuxR can bind to it's native or other AHLs and activates three promoters. Results
Modeling crosstalkEach experimental data set was fitted to an Hill function using the Least Absolute Residual method. The fitting of the graphs was performed using the following equation :
| ||||||||||||||||||||||||||||||||||||
|
•••••
SUN(Tsinghua) |
Part was sequenced and functional. LuxR was used in our Portable Pathogen Detector. |
|
•••••
wmholtz |
Using this part, I have successfully constructed and tested a quorum sensing circuit in E. coli. |
|
•••••
Youri |
This part was used and tested as a subpart in K546000, K546001, K546002, K546003, K546005 and K546546. This part functioned in all cases. |
|
Kevin (iGEM Braunschweig 2013) |
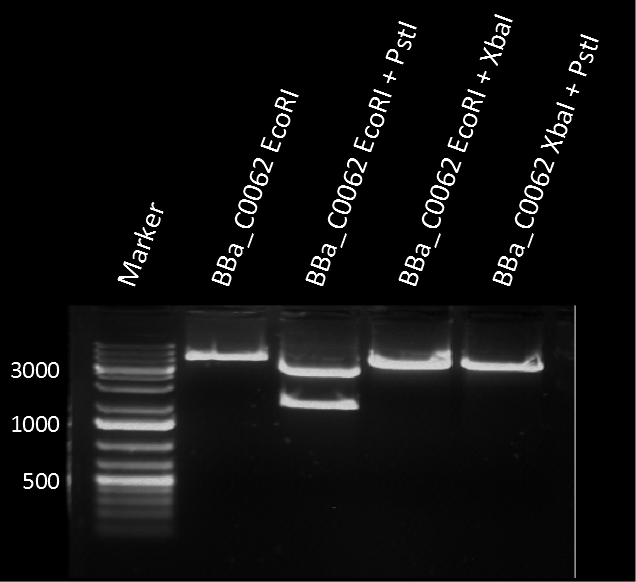
The plasmid pSB1C3 BBa_C0062 from the 2013 distribution Kit was transformed in E. coli XL1 BlueMRF. Sequencing with standard verification primer VF2 confirmed matching sequence of backbone DNA up to the EcoRI restriction site. The rest of the sequence (not shown) does not match the registry entry.
96 145
pSB1C3 LuxR (96) GAGGCAGAATTTCAGATAAAAAAAATCCTTAGCTTTCGCTAAGGATGATT
C0062 VF2 (1) GAGGCAGAATTTCAGATAAAAAAAATCCTTAGCTTTCGCTAAGGATGATT
146 195
pSB1C3 LuxR (146) TCTGGAATTCGCGGCCGCTTCTAGAGATGAAAAACATAAATGCCGACGAC
C0062 VF2 (51) TCTGGAATTCGACGCAA-TGGGTGCGCTGTCTACTAAATACAACGACACC
196 245
pSB1C3 LuxR (196) ACATACAGAATAATTAATAAAATTAAAGCTTGTAGAAGCAATAATGATAT
C0062 VF2 (100) CCGGAAAAAGCCTCCCGTACTTACGACGCTCACCGTGACGGTTTCGTTAT
246
pSB1C3 LuxR (246) TAATCAATGC...
C0062 VF2 (150) CGCTGGCGGC...
A restriction assay (Figure 1) showed that the sequenced part has no XbaI restriction site following the EcoRI site indicating another part in front of BBa_C0062 with a length of at least 1000 bp. |
UNIQ633255bcde4fd56d-partinfo-00000007-QINU