Part:BBa_K3757010
35S CtAEP1
Gene encoding the cyclizing asparaginyl endopeptidase 1 from Clitoria ternatea (BBa_K3757000), C-terminally tagged with HA-tag (BBa_K3183024). The gene is regulated by CaMV 35S promoter (BBa_K788000) and 35S terminator (BBa_K1159307). This part can be co-expressed with a peptide embedded in the cyclotide precursor composite part 35S Oak1 (BBa_K3757007) to yield this peptide in a cyclic form.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1882
Illegal BamHI site found at 2138
Illegal BamHI site found at 2309 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 66
Contents
Usage and Biology
This part can be used for the expression of the cyclizing asparaginyl endopeptidase CtAEP1 in plants, C-terminally tagged with a HA-tag. After expression, the included tag may be used for affinity purification or detection of the CtAEP1 with an anti-HA-tag antibody in an immunoassay. It inherits the following basic parts:
- CaMV 35S promoter (BBa_K788000)
- CtAEP1 (BBa_K3757000)
- HA-tag (BBa_K3183024)
- 35S terminator (BBa_K1159307)
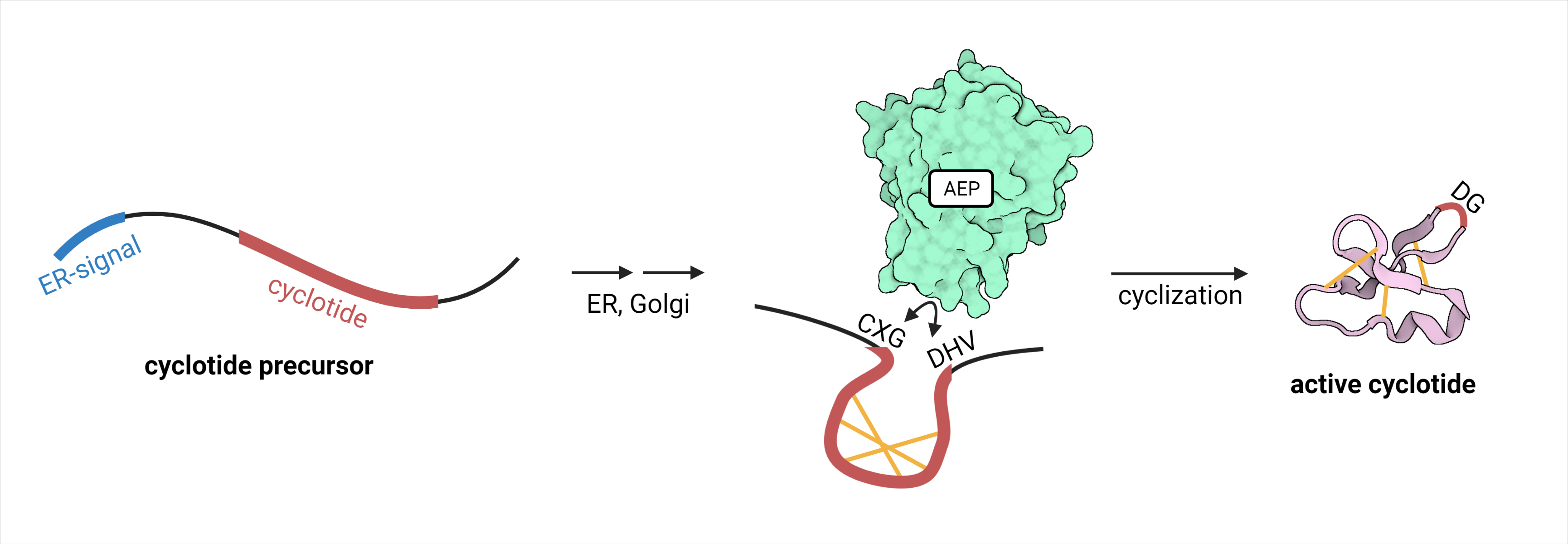
CtAEP1, also called butelase 1, is an Asx-specific ligase from the family of asparaginyl endopeptidases (AEP) (EC 3.4.22.34). It originates from the herb Clitoria ternatea. Compared to other endopeptidases, it does not only cleave peptide bonds, but it ligates two cleaved termini (figure 1). It therefore specifically recognizes substrates with the sequence “NHV” or “DHV” as a C-terminal propeptide. The sequence requirements for the N-terminal cleavage recognition site “CXG” are lower, requiring X not to be a proline residue. The peptide bond between N/D and HV is cleaved, linking the C-terminal N/D to the N-terminal G. With a catalytic efficiency of 542,000 M-1 s-1, CtAEP1 is the fastest protein ligase known. Its natural function is the cyclization of cyclotides, which are peptides involved in plants’ defense mechanism.1 CtAEP1 is expressed as an inactive precursor protein with a C-terminal cap-domain blocking its active site. After translation, it is localized to the vacuole by its N-terminal signal sequence. The low pH environment in the vacuole leads to autocatalytic cleavage of the cap-domain exposing the catalytic triad and thereby activating it. Cyclization and therefore activation of its substrate cyclotides takes place in the vacuole.2
CtAEP1 has been successfully used for the cyclization of proteins in vitro3. It was also demonstrated that CtAEP1 could lead to cyclization of cyclotides in Nicotiana benthamiana (N. benthamiana), when both the cyclotide precursor and the CtAEP1 were recombinantly coexpressed4.
Tuebingen 2021
iGEM team Tuebingen 2021 used CtAEP1 to express stabilized antimicrobial peptides (AMPs) in N. benthamiana. These peptides are grafted into a cyclotide scaffold, which can be backbone cyclized by the co-expressed CtAEP1. For further information, visit the project description wiki page of team Tuebingen 2021.
CtAEP1 was expressed in N. benthamiana, regulated by a CaMV 35S promoter (BBa_K788000) and a 35S terminator (BBa_K1159307), C-terminally tagged with a HA-tag (BBa_K3183024). This is summarized as the composite part BBa_K3757010. In 3in1 vectors (BBa_K3757011), the AEP was co-expressed with cyclotide constructs embedded in the Oak1 precursor (BBa_K3757001), and a GFP reporter gene (BBa_K3669012). Furthermore, 2in1 vectors were cloned, which contain only the genes for the Oak1 precursor and GFP, but not for CtAEP1. For further information, visit the experiments wiki page of team Tuebingen 2021.
Expression in Nicotiana benthamiana and Protein Extraction
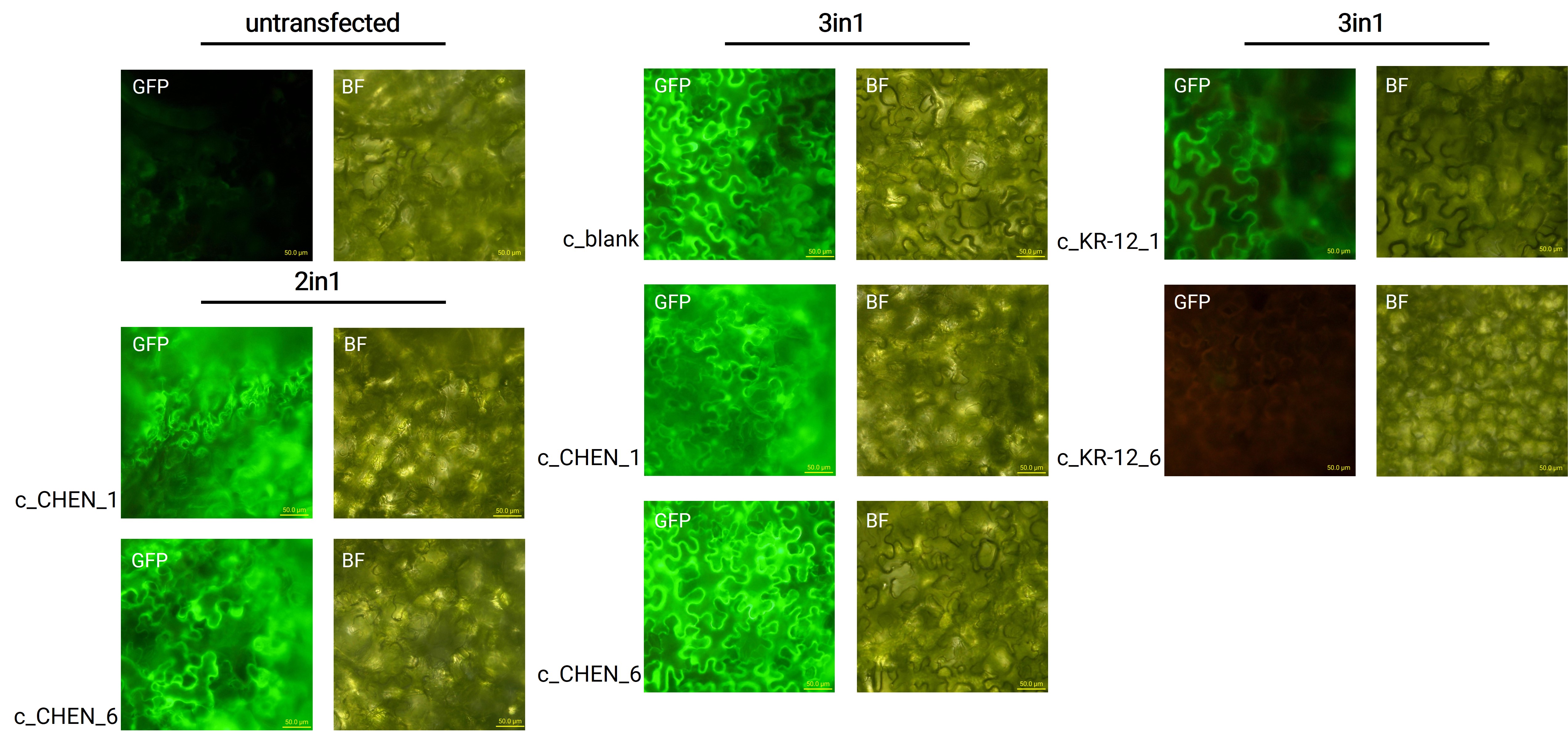
CtAEP1 was co-expressed with GFP (BBa_K3669012) as a reporter gene. Four days after agroinfiltration of N. benthamiana leaves, leaf pieces were observed with fluorescence microscopy (figure 2). This confirmed GFP expression. In comparison to the negative control, leaves transfected with our final vectors showed clear green fluorescence for all except the c_KR-12_6 construct (BBa_K3757006). As a negative control, non-infiltrated leaves or leaves infiltrated with agrobacterium p19 suppressor of gene silencing only were checked for green fluorescence. As shown in figure 2, this negative control showed no significant level of green fluorescence, confirming that the green fluorescence we detected for other vectors can only result from successful expression of one of our final vectors.
We can draw the conclusion that the transfection was successful for both the 2in1 and the 3in1 vectors. Our transfection strategy is efficient and works for CtAEP1 combined with different AMP constructs. However, not the whole infiltrated leaves were fluorescent but defined loci only, which indicates a low expression rate.
After homogenizing and extracting the harvested plant tissue with HEPES-buffer, a western blot was performed with the crude extracts. Primary rat anti-HA-tag antibody and secondary anti-rat HRP-coupled antibody were used, to detect the C-terminal HA-tag of the CtAEP1. However, we didn’t see any bands in the western blot (data not shown). Therefore, it is possible that the CtAEP1 is not expressed in our transfected N. benthamiana leaves. This would be unexpected, since CtAEP1 has been successfully transiently co-expressed with a cyclotide precursor in N. benthamiana before4. Furthermore, extraction of the AEP from the plant tissue or the anti-HA-tag western blot may not have worked properly. The anti-HA-tag antibody we used was reported to be a weak binder when using a single HA-tag. Therefore, it might be that CtAEP1 is present in amounts beneath the detection limit of the antibody we used.
In Vivo Cyclization Activity in Nicotiana benthamiana
Anti-His-tag Western Blots
The presence or absence of cyclized AMP-constructs would indicate indirectly whether the CtAEP1 is co-expressed and active in vivo.
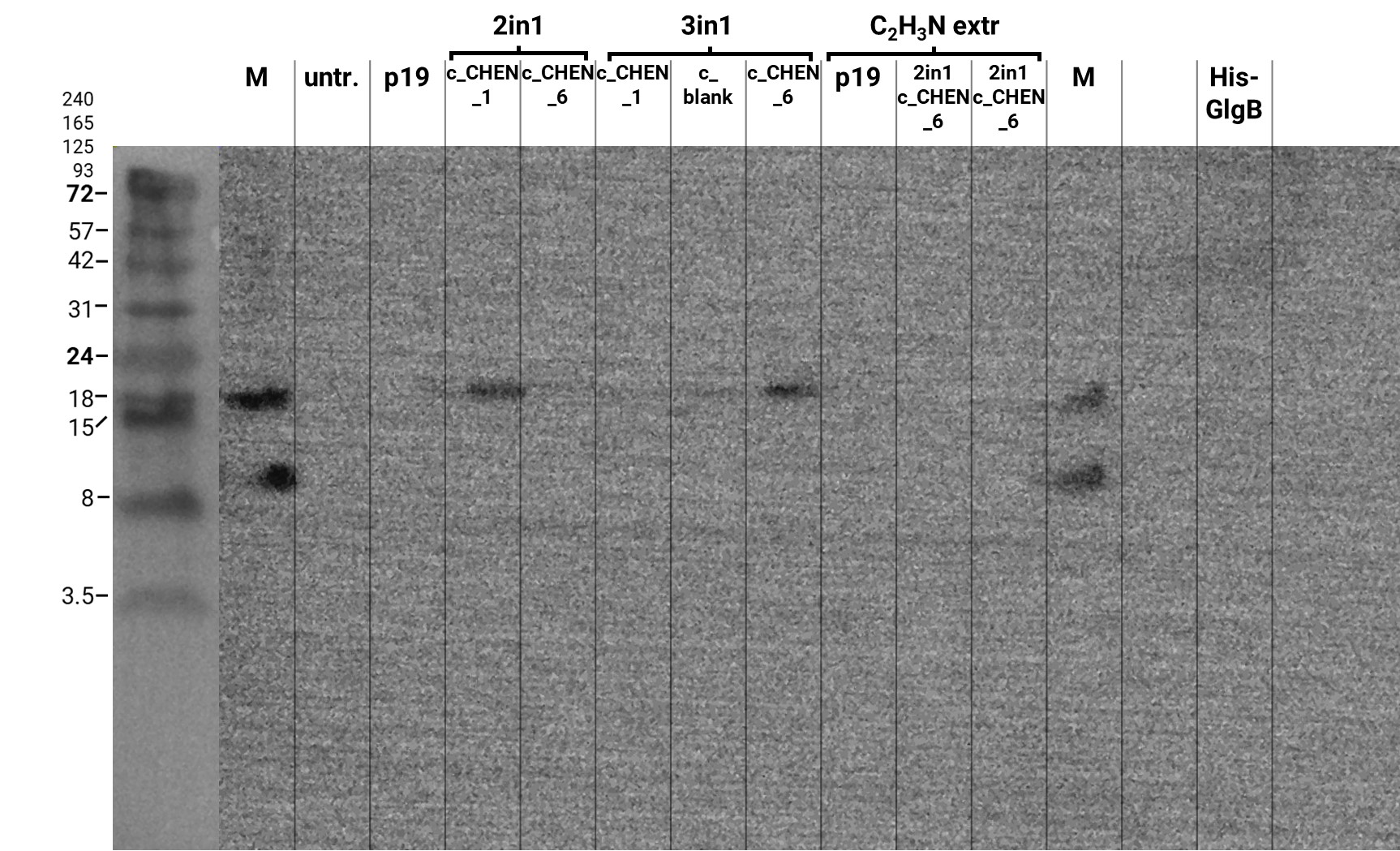
We performed a western blot of the very same crude extract samples used for the anti-HA-Tag western described earlier, with an anti-His-tag primary antibody to be able to specifically detect our His-tagged cyclotides. An immunoblot signal at a size of about 18 kDa could be detected (figure 3), which raised the suspicion that cyclization of our cyclotides by the AEP did not work, as the precursor of our constructs is about the same size as the detected signal. Another possible explanation is that the band shows indeed our cyclotide, which does not run at the expected molecular range in the SDS-PAGE. This could be due to its highly positive charge, which might not be completely masked by the negatively charged SDS or due to its cyclic structure. Unfortunately, no signal was detected for our positive control, a His-tagged protein of about 84 kDa. This could be explained by the huge size difference between this control and our peptide. Thus, it could have happened that the blotting of the control on the membrane did not work.
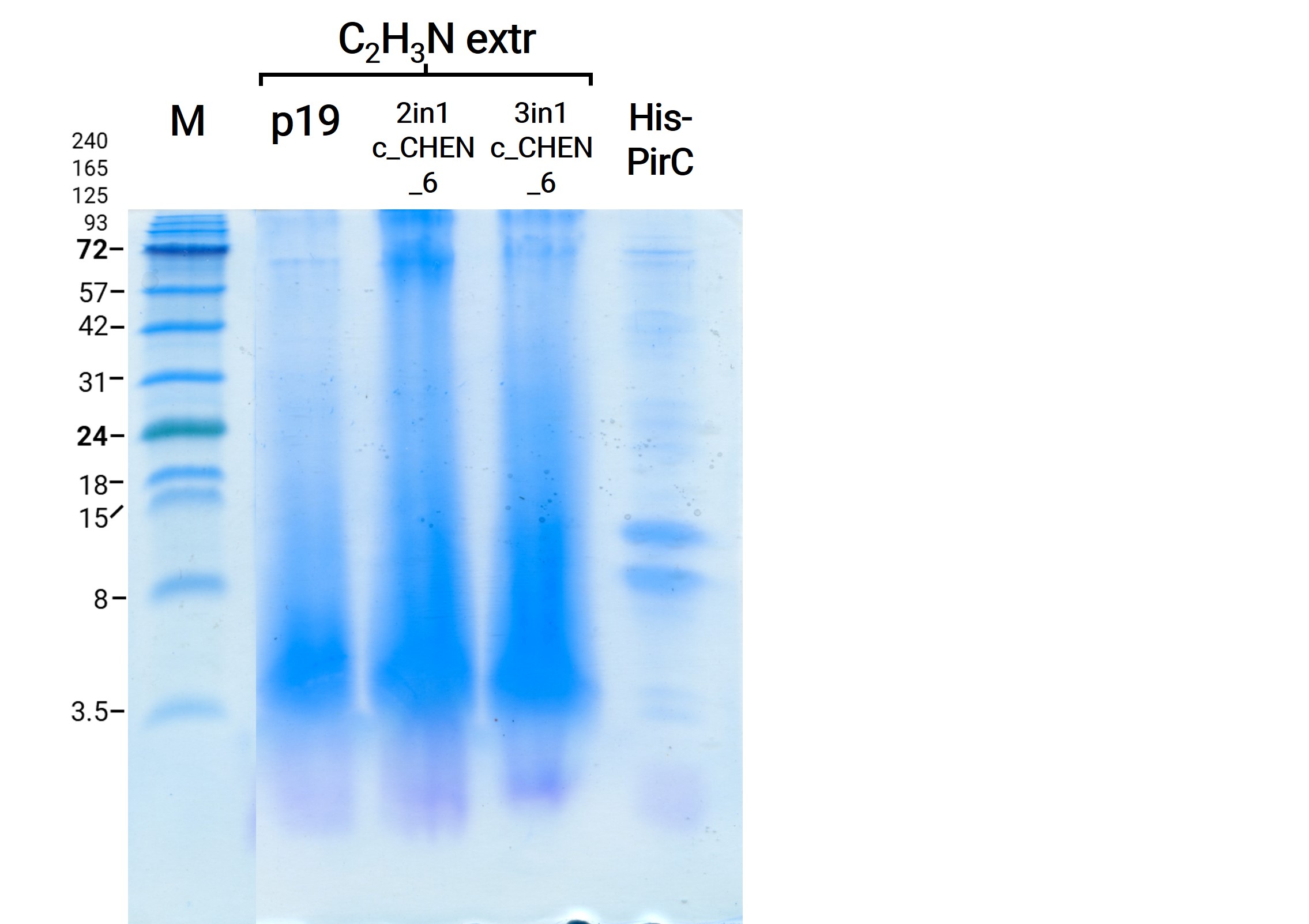
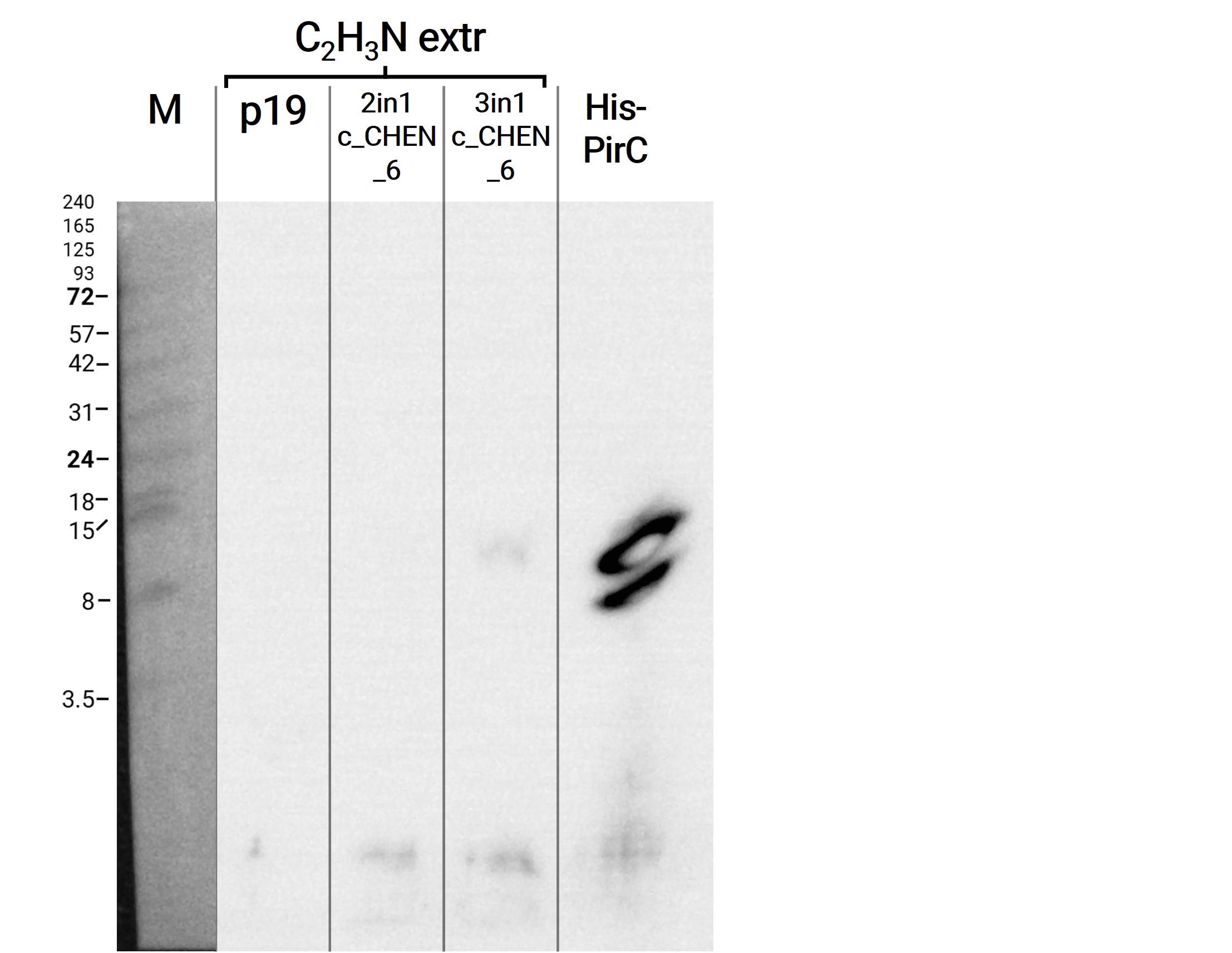
We then extracted leaves using an acidic-acetonitrile based buffer. For this method (adapted from Poon et al, 2018)4 we used dried leaf material and evaporated the extraction solvent in the last step. Prior to applying these samples onto the SDS-PAGE some of the extract was therefore solubilized in ddH2O and then mixed with 2X Lämmli buffer in equal ratio. As can be seen in figure 4, the lanes of these samples show an irregular picture, as no clear and distinct bands can be identified apart from one apparently quite big band at the height of 3.5 kDa. On an anti-His-tag western blot we were able to detect some signal (figure 5). However, the molecular weight (MW) range (smaller than 3.5 kDa) in which the signal occurs does not correspond to anything we expect. Only in the lane of the construct 3in1 c_CHEN_6 (BBa_K3757006) a weak band can be observed at the MW of about 8 kDa, which would be around the expected size of our cyclotide construct. This indicates that the CtAEP1, which is co-expressed in construct 3in1 c_CHEN_6 and absent in construct 2in1 c_CHEN_6, could be expressed and actively cyclizing the AMP-cyclotide precursor. But as the signal is very low, this assumption would have to be verified by further experiments.
MALDI-TOF
As stated above, plant crude extracts with Lämmli buffer and with acidic acetonitrile-based buffer showed protein bands with questionable identity: The 18 kDa band detected with an anti-His antibody in the Lämmli extracts, which may correspond to the cyclotide precursor or could indicate an unusual SDS-PAGE running pattern of the cyclotide itself, and the diffuse 3.5 kDa protein load visible in the Coomassie-stained acetonitrile extracts. To get more insights into the identity of these bands, mass spectrometry (MS) was performed.
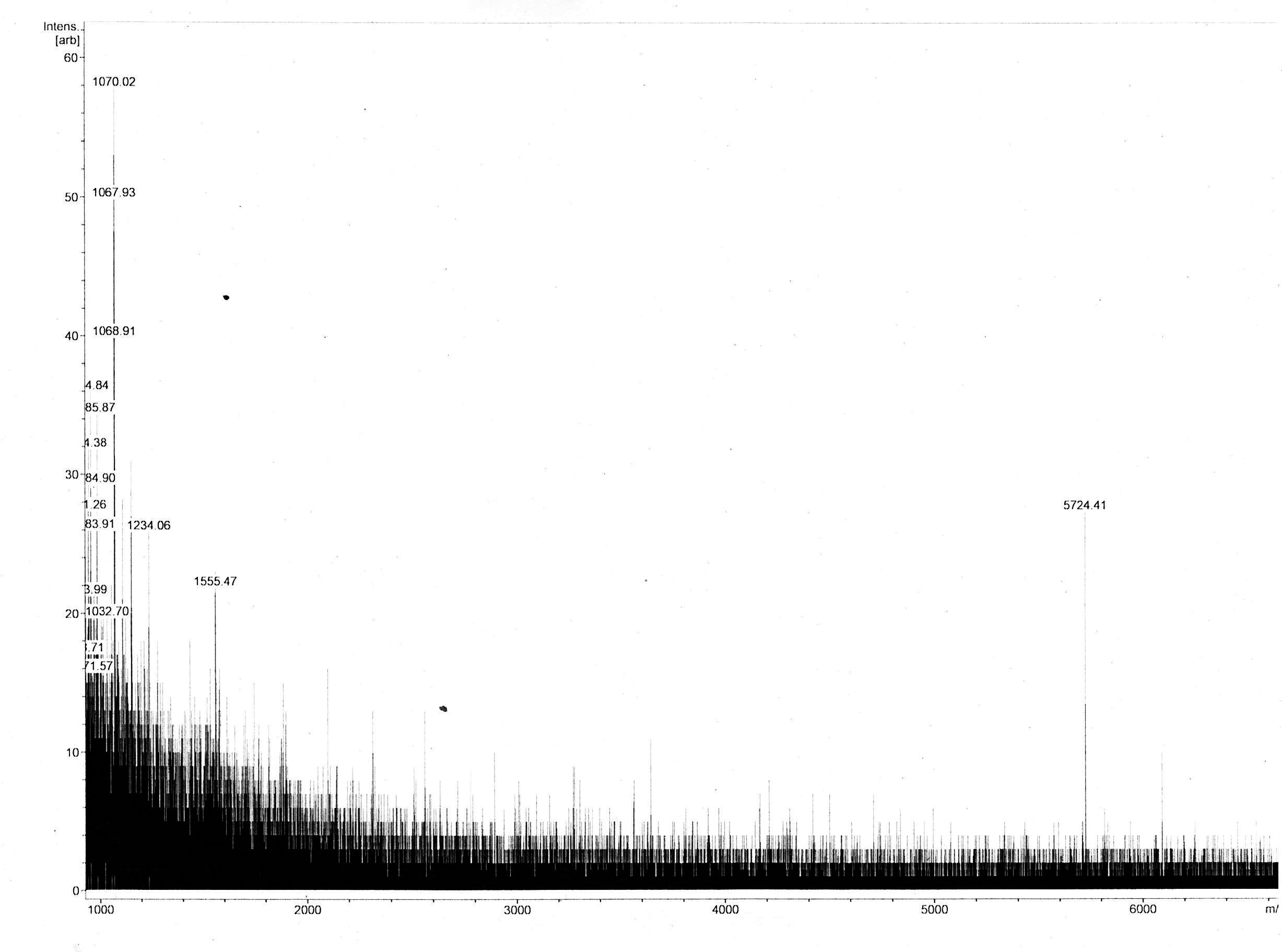
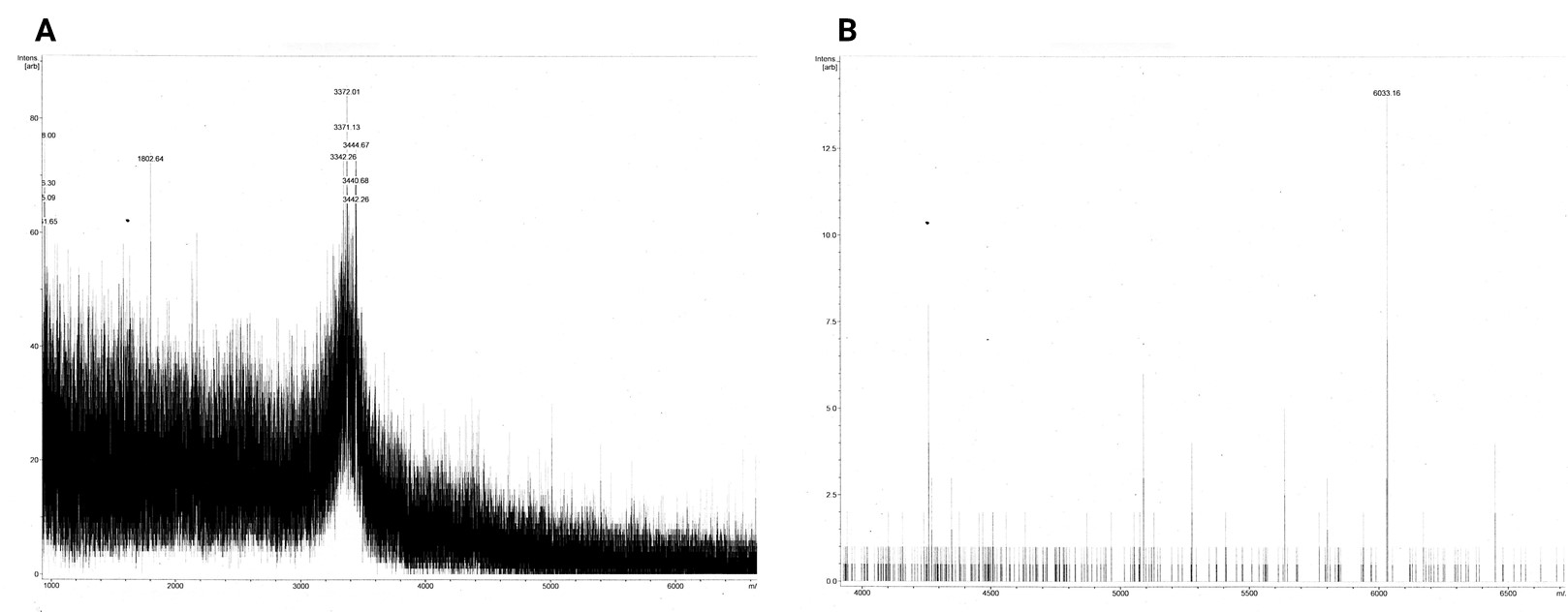
Most of the samples’ MS spectra showed no peaks with appropriate m/z ratio with relevant peak intensity. The MS spectrum of the 18 kDa Lämmli extract gel slice of 3in1 construct c_CHEN_1 (BBa_K3757003) showed a single peak corresponding to a m/z ratio of 5724.41 (figure 6). This value could fit with a single-charged M+ ion of cyclic c_CHEN_1, which has a theoretical average mass of 5718.30 Da with all cysteine (cys) residues reduced, as would be expected after the SDS-PAGE involving the reducing agent β-mercaptoethanol, and a theoretical average mass of 5712.30 Da with all cys residues oxidized. The deviation between theoretical mass and observed m/z ratio could be explained by the fact that the used mass spectrometer was only calibrated in the range of 2 to 4 kDa. Furthermore, it is unknown in which form our peptide occurs, since unknown modifications might be adhered. However, the running pattern in the SDS-PAGE gel does not fit the MW of our cyclotide construct. We speculated that the high positive charge of our construct, originating from its His6 tag and the cationic AMP graft, could influence its SDS running pattern.
The dissolved acetonitrile extracts (BBa_K3757004) were additionally concentrated and desalted using C18 resin. The MALDI-TOF MS spectrum of 2in1 construct c_CHEN_6 showed no fitting peaks. However, many peaks corresponding to m/z ratios around 3400 were visible (figure 7 A). These peaks fit single-charge M+ ions of peptides making up the broad band visible at around 3.5 kDa in the Coomassie-stained SDS-PAGE gel of the acetonitrile extracts. Therefore, we concluded that our cyclotides are unlikely to be represented by this 3.5 kDa band. The spectrum of 3in1 construct c_CHEN_6 showed a low-intensity peak with a m/z ratio of 6033.16 (figure 7 B). This value is similar to the average mass of a single-charged M+ ion of cyclic c_CHEN_6, which is expected to be 6041.89 Da with all cys residues reduced, and 6035.89 Da with all cys residues oxidized, as would be expected since no reducing agent was used on this construct.
Conclusion
All in all, the MALDI-TOF MS spectra show low-intensity peaks with m/z ratios quite similar to what would be expected for two of our cyclic AMP-cyclotide constructs. Together with the results obtained in the anti-HA-tag and the anti-His-tag western blots, we assume that the CtAEP1 is expressed in our N. benthamiana plants harbouring the 3in1 constructs. However, the CtAEP1 and the cyclotide precursors are either expressed, or are only isolated in very low amounts.
References
1Nguyen, G. K. T [Giang K. T.], Wang, S [Shujing], Qiu, Y., Hemu, X., Lian, Y., & Tam, J. P [James P.] (2014). Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis. Nature Chemical Biology, 10(9), 732–738. https://doi.org/10.1038/nchembio.1586
2James, A. M., Haywood, J., Leroux, J., Ignasiak, K., Elliott, A. G., Schmidberger, J. W., Fisher, M. F., Nonis, S. G., Fenske, R., Bond, C. S., & Mylne, J. S. (2019). The macrocyclizing protease butelase 1 remains autocatalytic and reveals the structural basis for ligase activity. The Plant Journal : For Cell and Molecular Biology, 98(6), 988–999. https://doi.org/10.1111/tpj.14293
3Nguyen, G. K. T [Giang K. T.], Kam, A., Loo, S., Jansson, A. E., Pan, L. X., & Tam, J. P [James P.] (2015). Butelase 1: A Versatile Ligase for Peptide and Protein Macrocyclization. Journal of the American Chemical Society, 137(49), 15398–15401. https://doi.org/10.1021/jacs.5b11014
4Poon, S., Harris, K. S., Jackson, M. A., McCorkelle, O. C., Gilding, E. K., Durek, T., van der Weerden, N. L., Craik, D. J [David J.], & Anderson, M. A. (2018). Co-expression of a cyclizing asparaginyl endopeptidase enables efficient production of cyclic peptides in planta. Journal of Experimental Botany, 69(3), 633–641. https://doi.org/10.1093/jxb/erx422
| None |