Part:BBa_K3182001
Contents
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 511
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Introduction
CBDcipA-GS+Thrombin linker
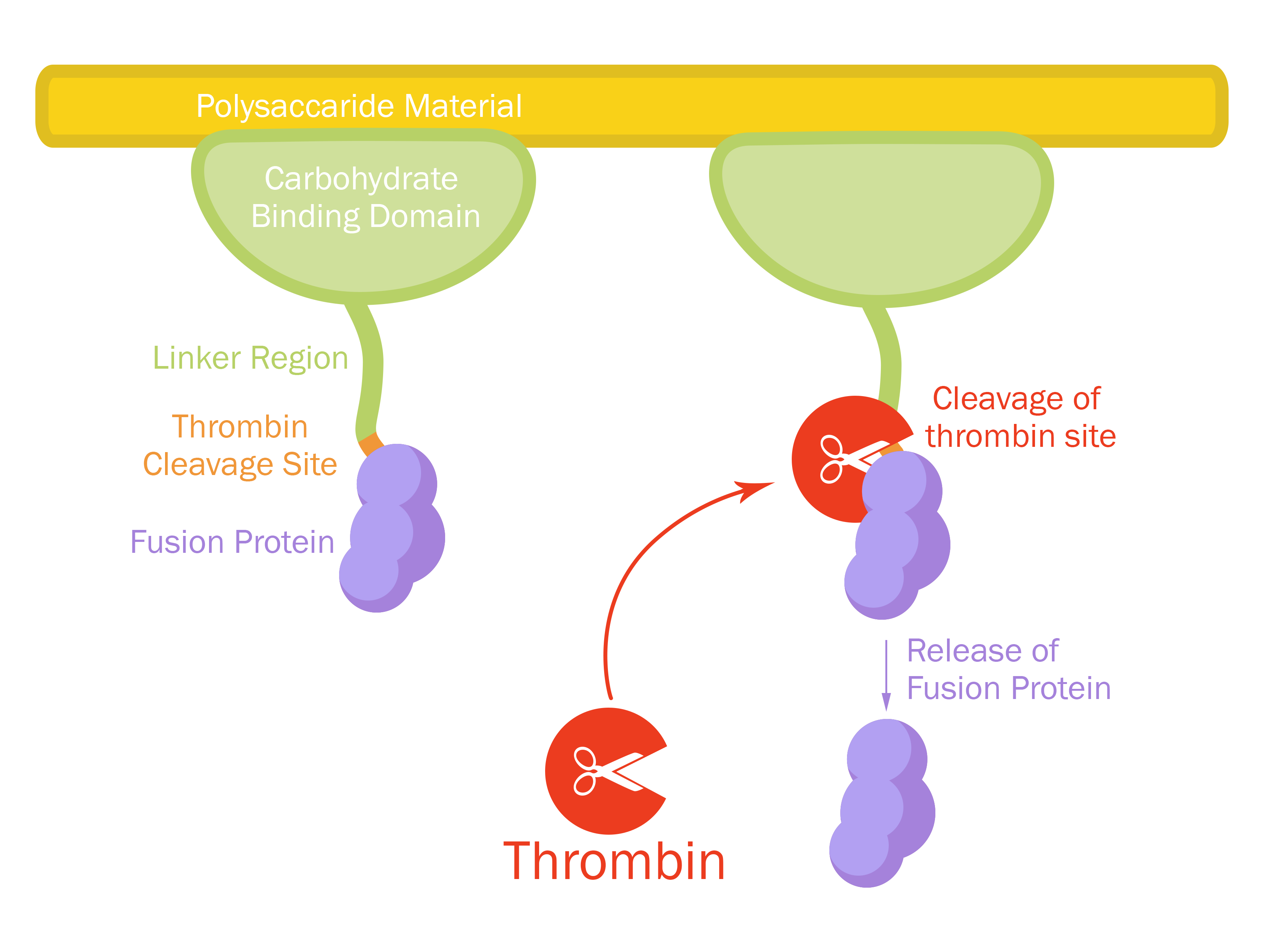
This part consists of a carbohydrate binding domain (CBD) from Clostridium thermocellum (C. thermocellum) cellulose scaffolding protein (CipA). This binding domain is a central part of Clostridium thermocellum's cellusome and has a strong affinity for cellulose. The CBD was fused to another protein using a flexible GS-linker (-GGGGSGGGGS-)in order to attach this complex to a polysaccaride material. A thrombin cleavage site (-LVPRGS-) was added to the end of the linker and its breakage will leave a glycine and serine attached to the N-terminal of the fusion protein. The main mechanism of iGEM19 Linköping's project can be seen in Figure 1.
Protease site and use
The thrombin site was added to enable the ability to release the fusion protein down into skin wounds. Thanks to our integrated human practice we learned that infections span much deeper into wounds that we thought. Simply attaching the CBD-fusion protein to a carbohydrate material would not enable the fusion protein to reach far into the wound. The thrombin site was also chosen because of thrombin's endogenous existence in humans.
Assembly compabilities
An internal BamHI recognition sequence (RS) has been added to enable interchangeable fusion proteins to the CBD. BamHI was chosen because its RS codes for glycine and serine, fitting it to the end of the thrombin site. It is also a cost-effective enzyme and is unaffected by methylated DNA. BamHI is a part of the RFC21 standard.
CBDcipA crystal structure
Important molecular faces
CBDcipA is composed of a nine-stranded beta sandwich with a jelly roll topology and binds a calcium ion, which can be seen in Figure 2. It further contains conserved residues exposed on the surface which map into two clear surfaces on each side of the molecule. One of the faces mainly contains planar strips of aromatic and polar residues which may be the carbohydrate binding part. Further aspects are unknown and unique to this CBD such as the other conserved residues which are contained in a groove.
Carbohydrate binding domain specificity
Since the CBD is from the cellusome of C. thermocellum some research labeled it a cellulose binding domain. However, iGEM19 Linköping noticed that this domain could also bind to different sources of polysaccaride materials. This serves as a domain for iGEM19 Linköpings modular bandage, where the polysaccaride material can be exchanged for other/similar materials and not exclusively cellulose.
The choice of carbohydrate binding domain
iGEM Linköping 2019 chose CBDcipA due to the fact that many other iGEM teams had explored the possibilities of this domain. Our basic design was influenced by [http://2014.igem.org/Team:Imperial iGEM14 Imperial], [http://2015.igem.org/Team:edinburgh iGEM15 Edinburgh] and [http://2018.igem.org/Team:ecuador iGEM18 Ecuador]. Purification and where to place the fusion protein (N- or C-terminal) was determined by studying the former projects. CBDcipA also originates from a thermophilic bacteria which further increases the domain's applications.
Expression system
The part has a strong expression with a T7-RNA-polymerase promotor (BBa_I719005), seen in Figure 3, as well as a 5'-UTR (BBa_K1758100) region which has been shown to further increase expression in Escherichia coli (E. coli) (BBa_K1758106), ([http://www.ncbi.nlm.nih.gov/pubmed/2676996 Olins et al. 1989]), ([http://www.ncbi.nlm.nih.gov/pubmed/23927491 Takahashi et al. 2013]).

Usage and Biology
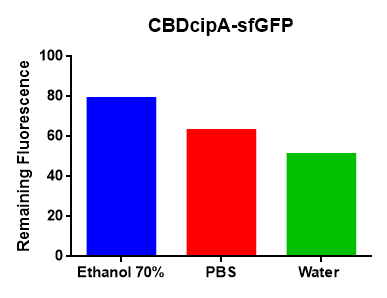
Figure 4. Picture left: Lysate (via sonication) from BL21 E. coli was incubated with Epiprotect for 1h and washed thrice with 70% ethanol. To the right is Epiprotect that has not been incubated with AsPink-CBD lysate. CBD-binding reported with: BBa_K3182000. Picture middle: Lysate (via sonication) from BL21 E. coli was incubated with cellulose fibres (Whatman CF-11 Fibrous Medium Cellulose Powder). Saturated cellulose was incubated with different units (U) of thrombin (Novagen, #69671).The leftmost is cellulose with cleavage buffer and the control contains bound AsPink with cleavage buffer. All tubes has been incubated over-night in R.T on an end-to-end rotator. CBD-binding reported with: BBa_K3182000. Picture right: Binding studies of the CBDcipA-sfGFP bound to bacterial cellulose. Washed three times with either 70 % ethanol, PBS or deionized water. CBD-binding reported with: BBa_K3182108.
Reporting CBD characteristics with pT7-CBDcipA-sfGFP (BBa_K3182108)
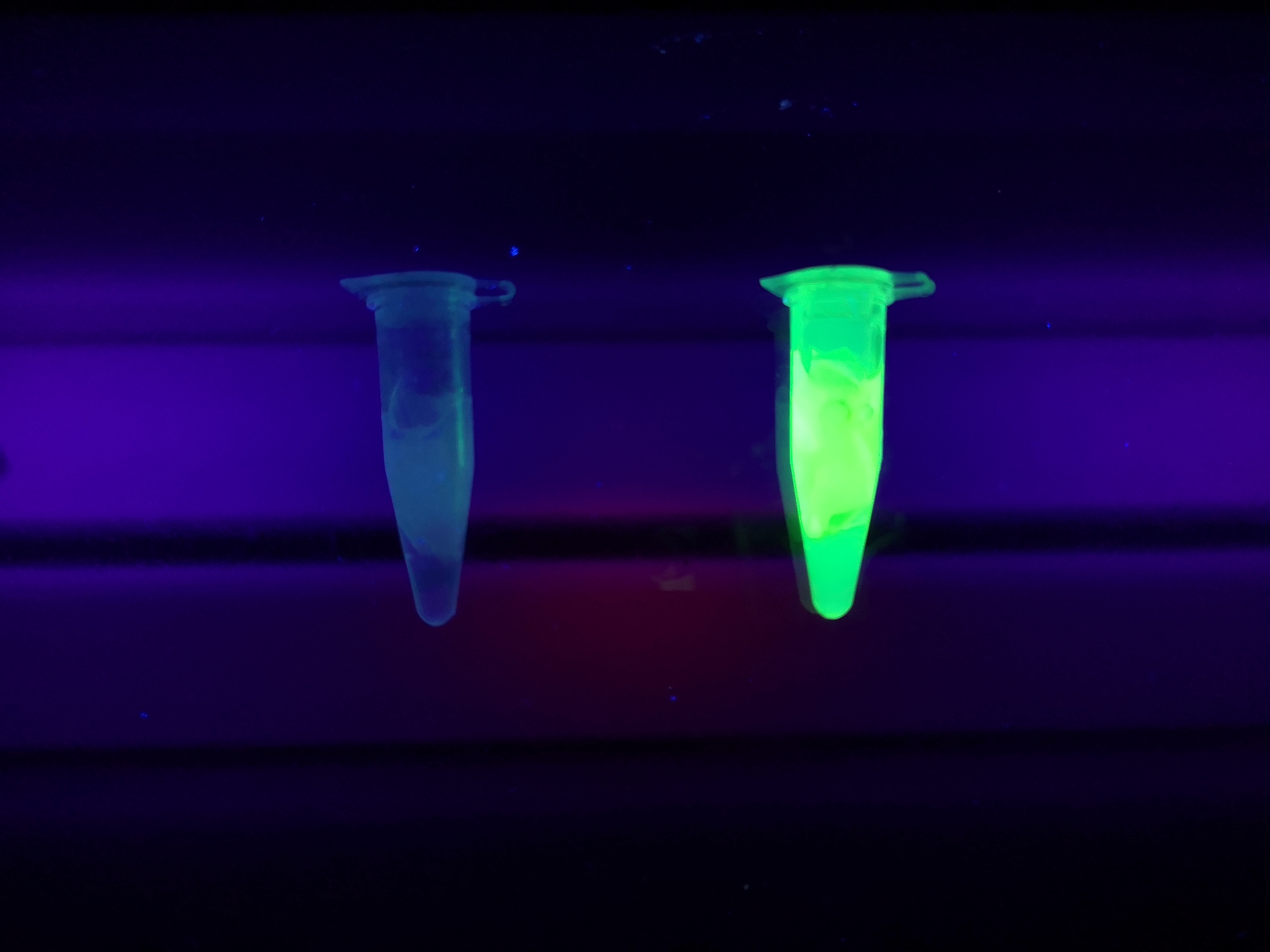
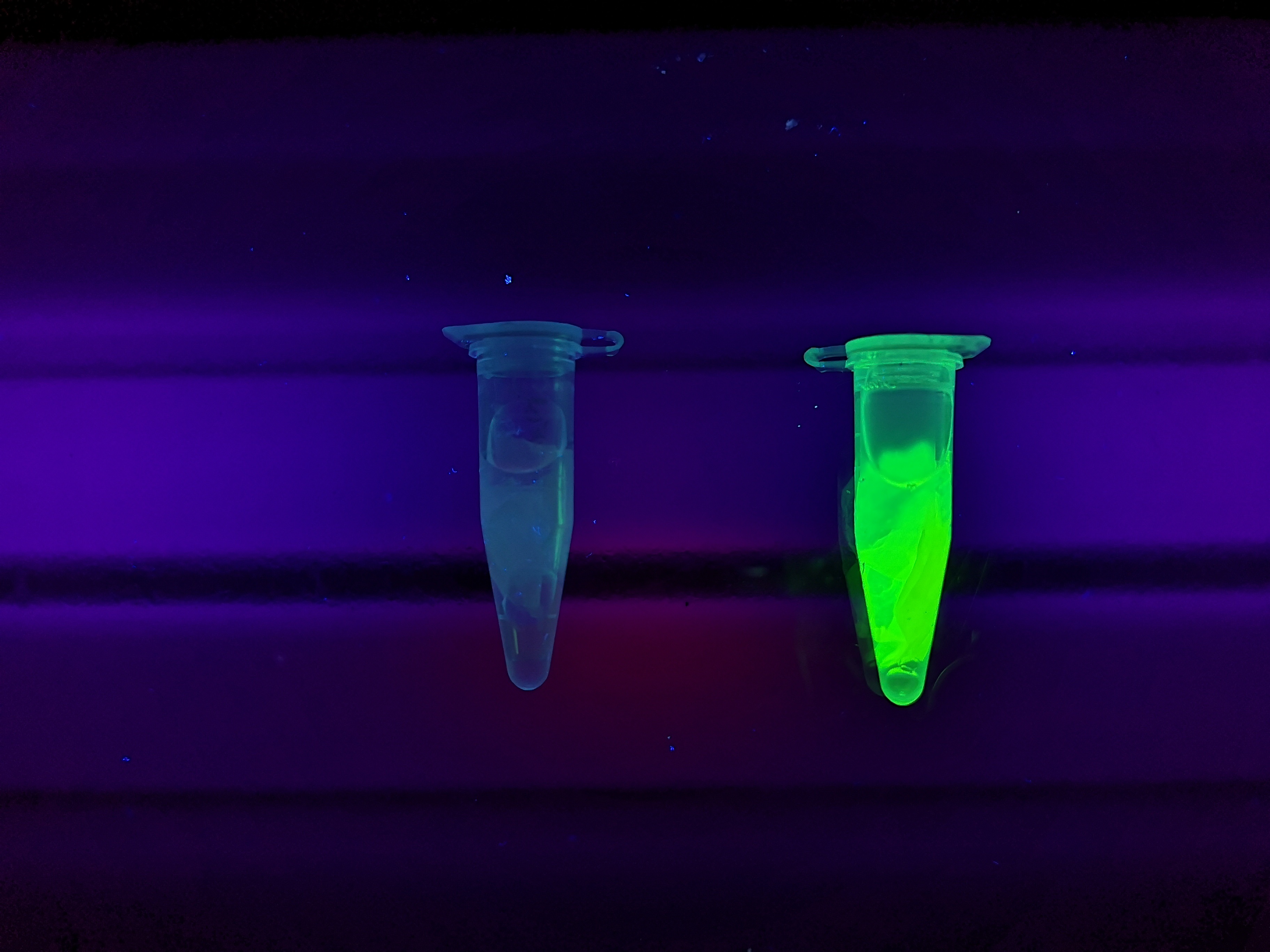
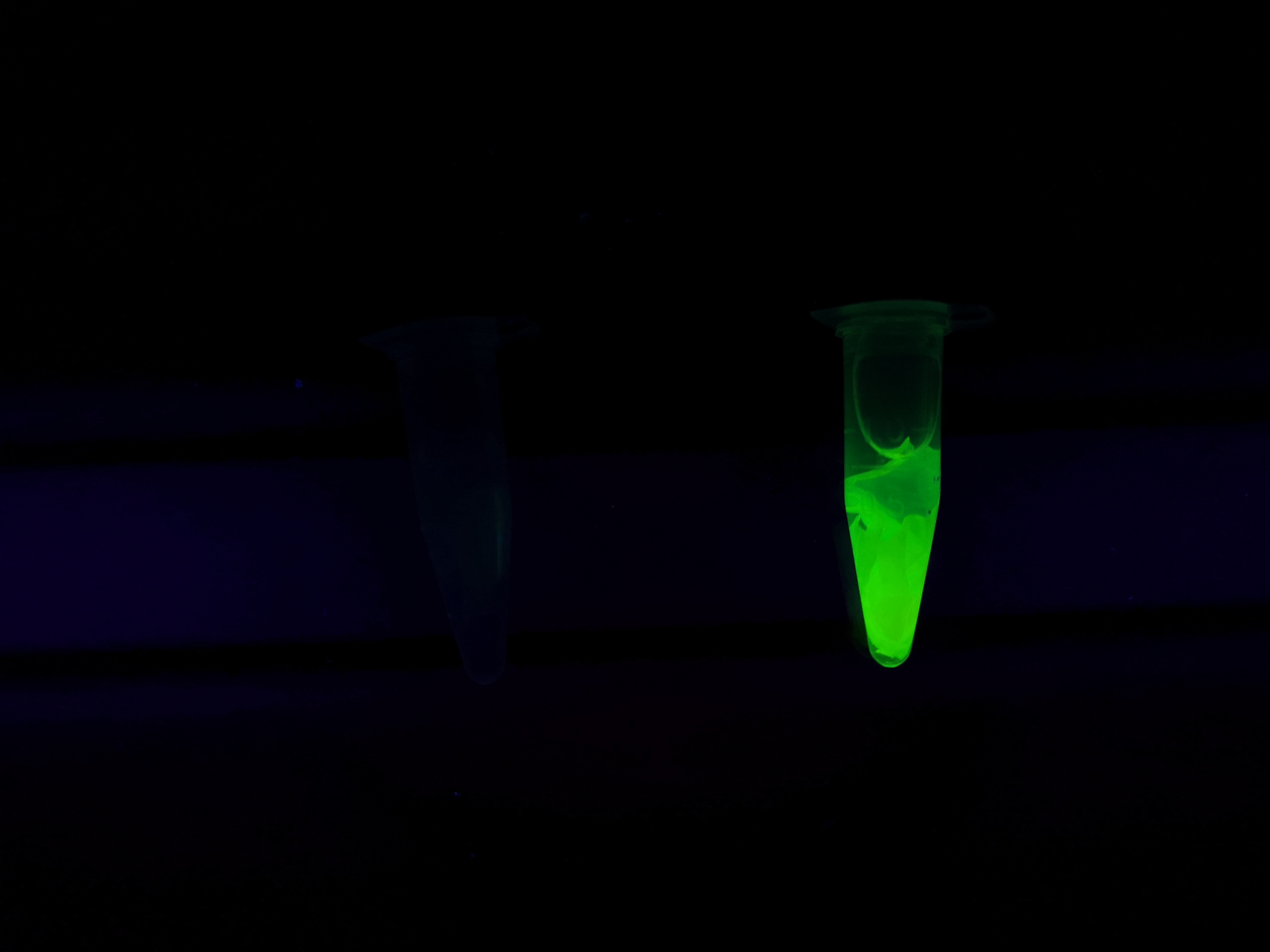
Figure 5 Picture 1: Lysate containing CBDcipA-sfGFP with bacterial cellulose (Epiprotect, S2Medical) before incubation. Picture 2: Lysate (CBDcipA-sfGFP) bound to bacterial cellulose after incubation in room temperature for 30 minutes on an end-to-end rotator. Picture 3: Bacterial cellulose after incubation with 70 % ethanol in room temperature for 30 minutes on an end-to-end rotator. All pictures were taken on a 302 nm UV-table for better visualisation of the result.
In figure 6 the same BL21CBD-sfGFP lysate was used and the samples were incubated the same amount of time, 30min in room temperature. Both the CBD-sfGFP bound agarose and agarose that was not incubated CBD-sfGFP was washed in 70% ethanol once. The agarose with CBD-sfGFP bound achieved a yellow tint in comparison to the agarose with no CBD-sfGFP which was the original transparent color.
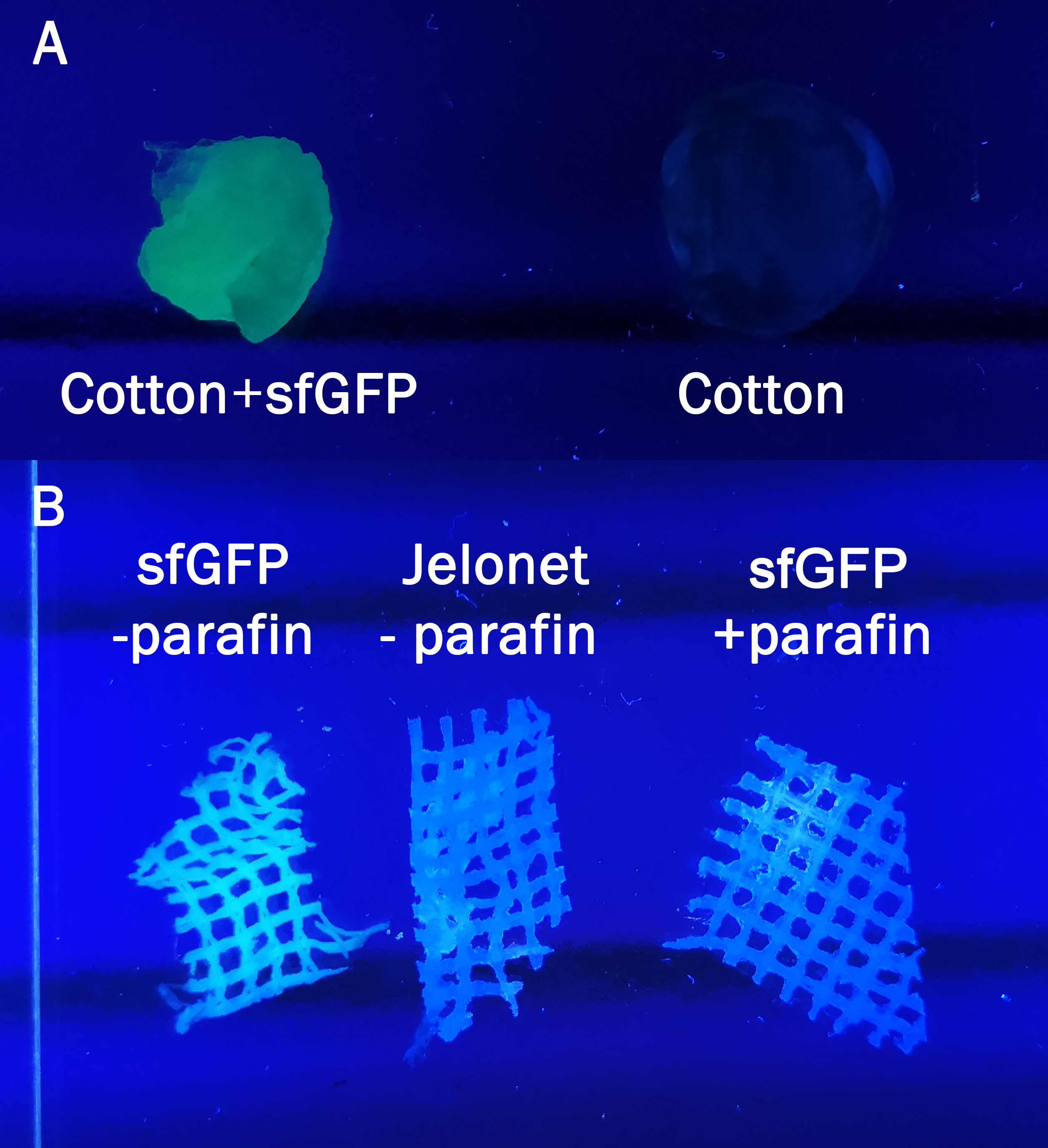
The samples in figure 7 were incubated in the same BL21 (DE3) lysate for 30 min and then washed three times with 70% ethanol. In A normal cotton was incubated in sfGFP whilst in B a bandage called jelonet was used. Jelonet contains cotton threads that are covered in paraffin. We discovered that the paraffin inhibited the binding of CBD-sfGFP, therefore using detergent we removed it for the CBD-sfGFP incubation.
All of the figures above are to illustrate the modularity of the CBD, proving that it does not only bind to cellulose such as in the first figures, but also to other polysaccharide containing materials. By binding CBD-sfGFP to jelonet we aimed to prove that the construct can be bound to already existing materials in the medical field. Jelonet is commonly used for open wounds such as burn wounds, both in order to create some antibacterial barrier and also to prevent the wounds sticking to gauze used above jelonet.
The bacterial cellulose bandage used in figures 4 and 5 is named Epiprotect and is produced by S2Medical. Epiprotect is currently used for wounds such as burn wounds and chronic wounds. These tests were also performed to prove that the construct can be used in existing medical products. In figures 11, 12 and 13 Epiprotect was used to observe ability of thrombin cleavage of the construct.
Purification of CBD-sfGFP
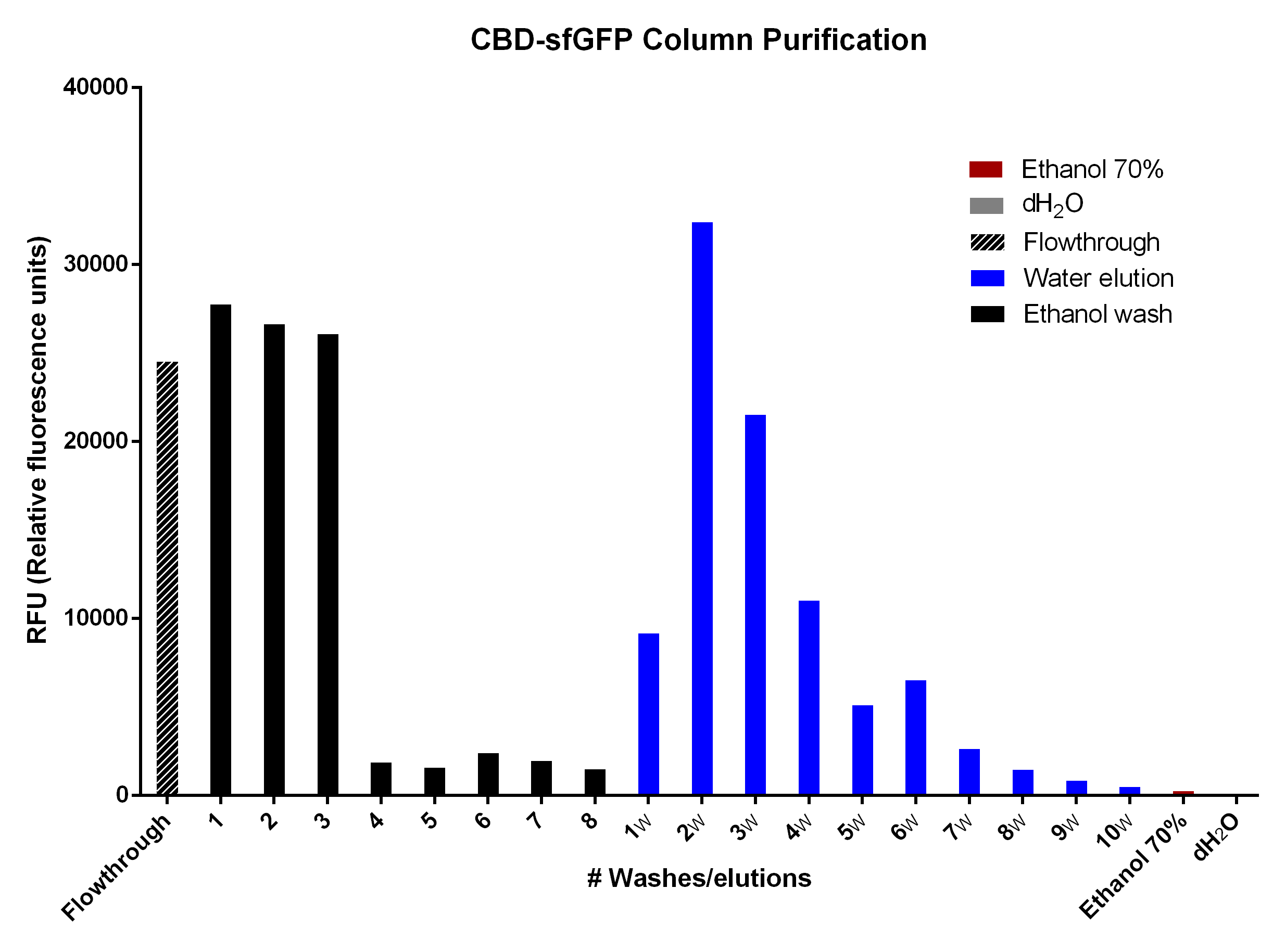
CBD-sfGFP was purified with 2g cellulose fibres medium length (Whatman, #CF11 medium length cellulose fibres) in a column. The first striped graph is the flowthrough after adding BL21 (DE3) Gold lysate.
The different washes of 70% ethanol (black bars) was to remove unspecifically bound CBD-sfGFP, leading to the bound CBD-sfGFP remaining. This can be seen in the bar graph due to the drastic drop of sfGFP elution which means that the 70% ethanol is not useful for CBD-sfGFP elution. The water washes (dH2O, blue bars) indicate that an efficient elution of CBD-sfGFP can be performed within the first 4-6 elutions of dH2O. The last two bars (red, ethanol 70% and grey, dH2O) indicate the endogenous fluorescent abilities of each solution. It is minimal for ethanol and none for dH2O.
The washes and elutions can be observed in an SDS-gel in figure 13 below. In which the lanes 3-6 contains the four washes with 70 % ethanol and lanes 7-10 contains the four first elution fractions with dH2O.
Binding assay of CBD-sfGFP
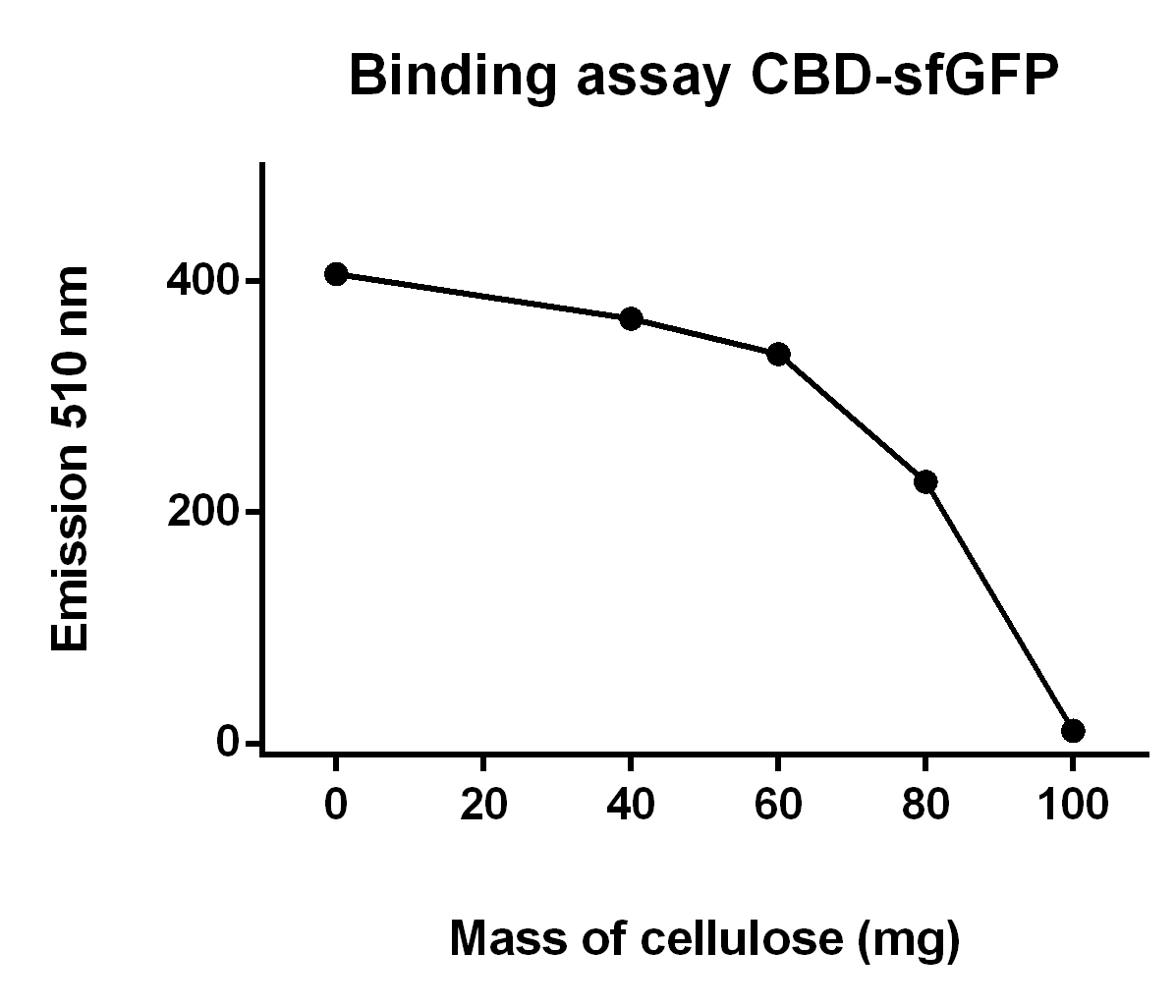
The illustration to the left (figure 9), shows that increasing mass of cellulose CF11 fibers leads to elevated the of CBD-sfGFP.
To make the binding assay, Eppendorf tubes with varying mass of cellulose (Whatman, #CF11 medium length cellulose fibres) between 0 - 100 mg was mixed with a constant volume of CBD-sfGFP lysate (700 uL) and vortexed. The tubes was then attached to an end-to-end rotator for 30 minutes, thereafter they were placed in a tube holder until the cellulose powder settled in the bottom and the supernatant containing lysate was clear of cellulose.
After that 100 µL from each of the supernatants was applied on a plate reader which measured the fluorescence of CBD-sfGFP at 485 nm.
Reporter of successful cleavage and release from the cellulose binding domain
Spectrophotometrically analysis of thrombin cleavage
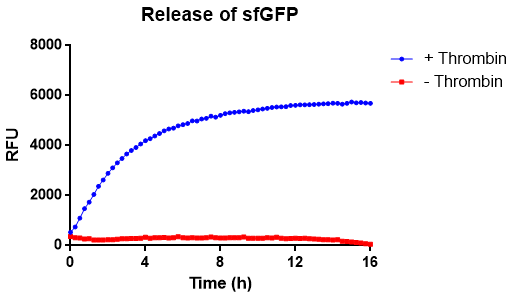
In figure 10 the release of sfGFP from our bacterial cellulose bandage (Epiprotect, S2Medical) can be seen over time. The approximate area och the cellulose was 1cm^2. The cellulose-CBD-sfGFP were attached to the side of wells of a 96-well plate using strings, allowing the spectrophotometer to measure the supernatant in the center of the well, bottom up (ex. 485 nm, em. 510 nm) for 16 hours. The well further contained 200 uL 1X thrombin cleavage buffer (20 mM Tris-HCl, 150 mM NaCl and 2.5 mM CaCl2). In the positive sample, measuring thrombin cleavage (blue), an amount of 0.03 units of human thrombin (Novagen, #69671-3) were added. In the graph (blue), successful release of sfGFP from the CBD can be seen. The maximun amount of fluorescence is reached after about 8h. In red, the negative control experiment can be seen, where thrombin buffer was added it contained no thrombin. No significant amount of flouorescence can be observed. The temperature was set to 37 °C. A visual representation of this can be observed below in figure 11.
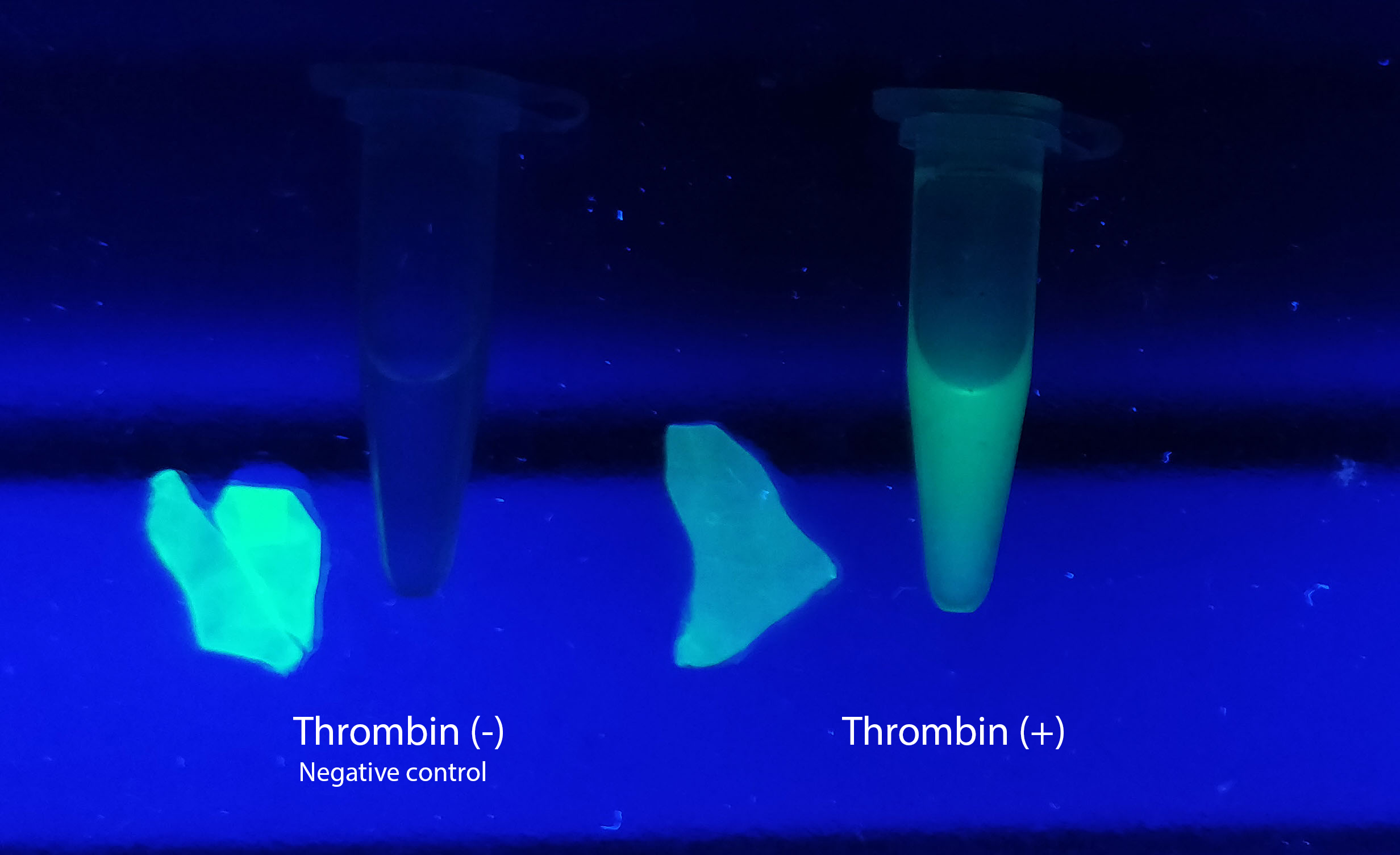
Visual experiment of thrombin cleavage
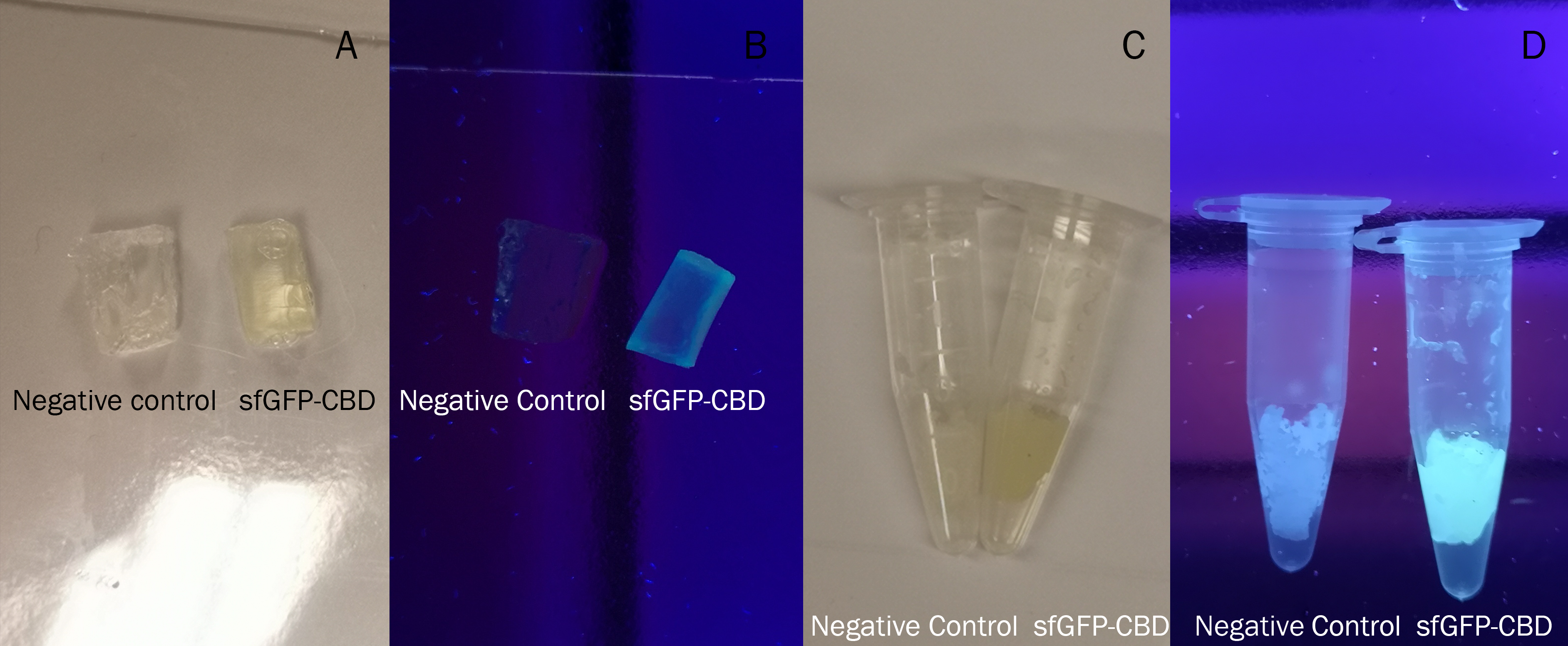
To the left a visual experiment with this part can be seen. After unbound protein had been removed the cellulose was washed three times with 70 % ethanol. To test the activity, 200 uL thrombin cleavage buffer (20 mM Tris-HCl, 150 mM NaCl and 2.5 mM CaCl2) were added along side 0.03 units of human thrombin (Novagen, #69671-3) to the bacterial cellulose (Epiprotect, S2Medical). To the right in the figure, the successful cleavage of CBD-sfGFP can be seen. The cellulose is to the left of the tube where free (cleaved at the thrombin site) sfGFP can be seen. To the left, the control sample can be seen, where no sfGFP can be seen in the supernatant. The picture is taken on a 302 nm UV-table to better visualise the results. Both samples were incubated in room temperature over-night on an end-to end rotator.
The thrombin containing sample (thrombin (+)) has a clear cleavage of the linker which can be observed in the tube's supernatant whilst the negative control does not have any sfGFP in its supernatant. Not all sfGFP has been cleaved from the cellulose bandage, this is probably due to the thrombin buffer running out of needed content for the thrombin cleavage.
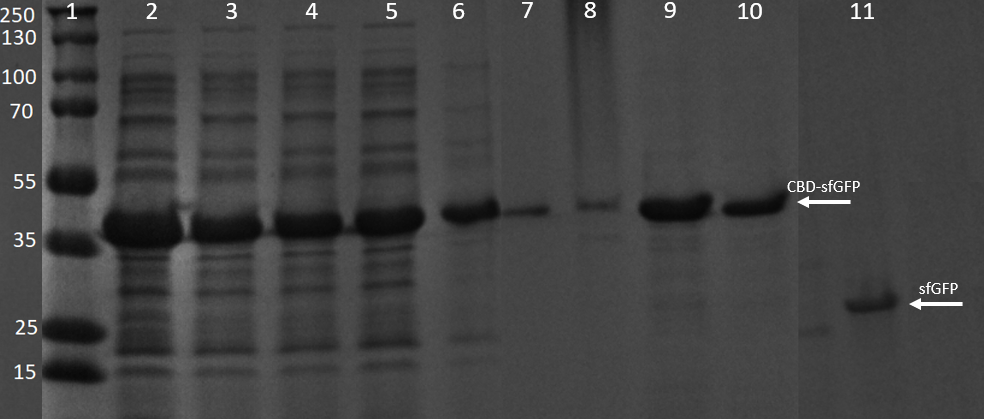
SDS-PAGE analysis of cleavage and expression
E. coli BL21 (DE3) Gold cells were grown in presence of 25 ug/mL chlorampenicol until an OD600 of 0.8 at 37 °C, and later induced with 0.5 mM IPTG. The induced culture were then incubated in 18 °C for 16 hours. The bacteria was then lysed with sonication at 30 % for 6 minutes. Most of this part could be found in the soluble fraction. The lysate (1 mL) was then incubated with cellulose (CF11) for 30 minutes in room temperature. Four washes with 70 % ethanol was then conducted all with a volume of 1 mL. Elution of CBDcipA-sfGFP was done with 1 mL fractions of dH2O. One replicate was instead cleaved with thrombin instead. Thrombin cleavage buffer (20 mM Tris-HCl, 150 mM NaCl and 2.5 mM CaCl2) was added to the Eppendorf tube with the cellulose at a volume of 500 uL, and 0.03 units was then added to the solution. The cleavage was done in room temperature over 16 hours, with inversion of the tube.
Other bacterial proteins are washed away with the ethanol which can be observed by the other fragments observed in the 3-6 lanes, all of the proteins can also be observed in the bacterial lysate lane 2. In lanes 7-10 water (dH2O) elutions can be observed, they also contain some of the other bacterial proteins though they are very faint and the last lane is almost only CBD-sfGFP. These elutions were the same as those in figure 7. Lane 11 contains only sfGFP which has been cleaved from the CBD construct, proving a successful cleavage of the linker, a photo of this cleavage can be seen in figure 11.
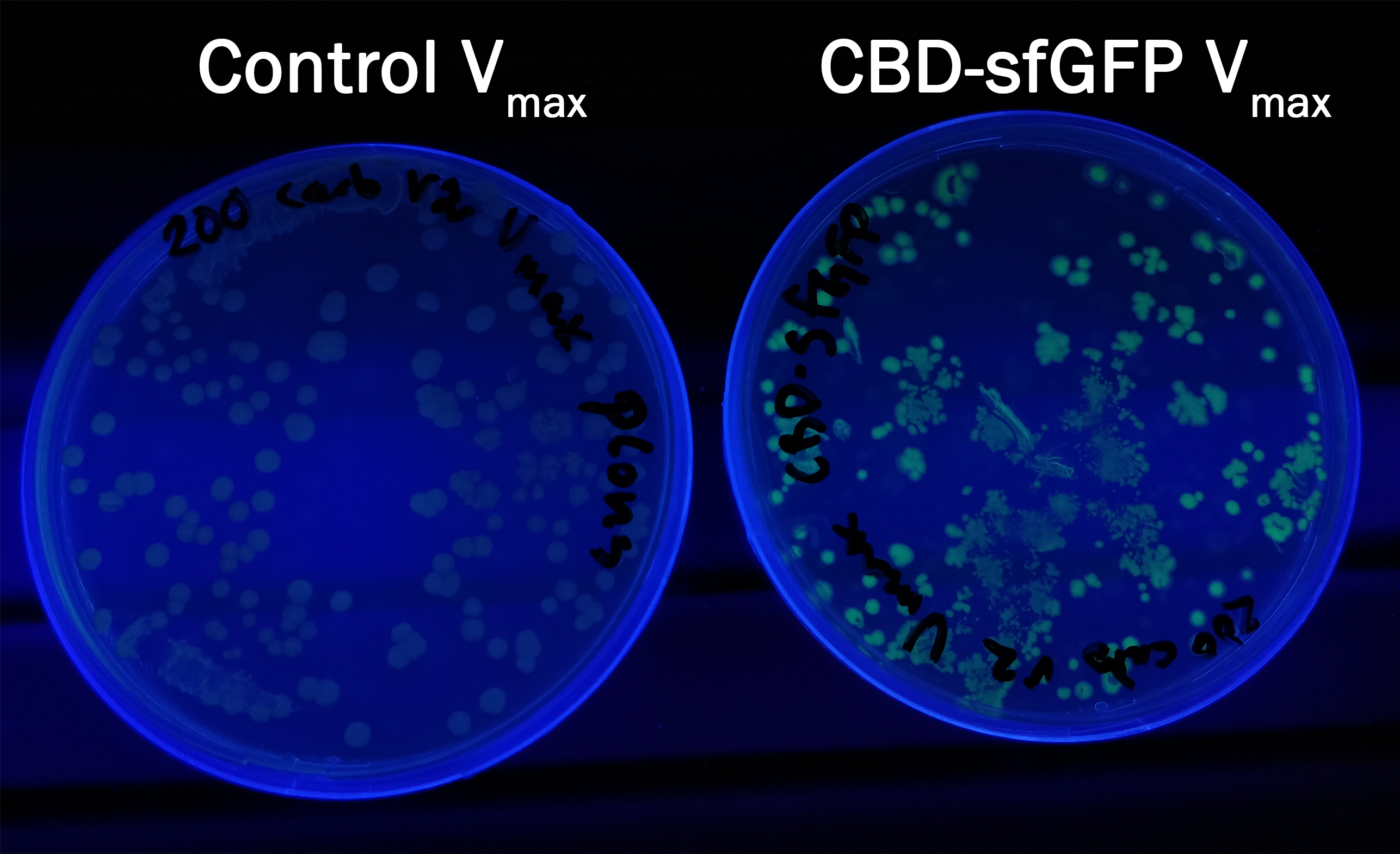
Expression in Vibrio Natriegens
The biobrick was heat shocked into V.natriegens and the culture was spread out on an LB-agar dish and allowed to grow for 16 h in 37 °C. V.natriegens
| None |