Part:BBa_K316045
LytC - Glycine Linker - Elastase Cleavage Site - AIP - His Tag
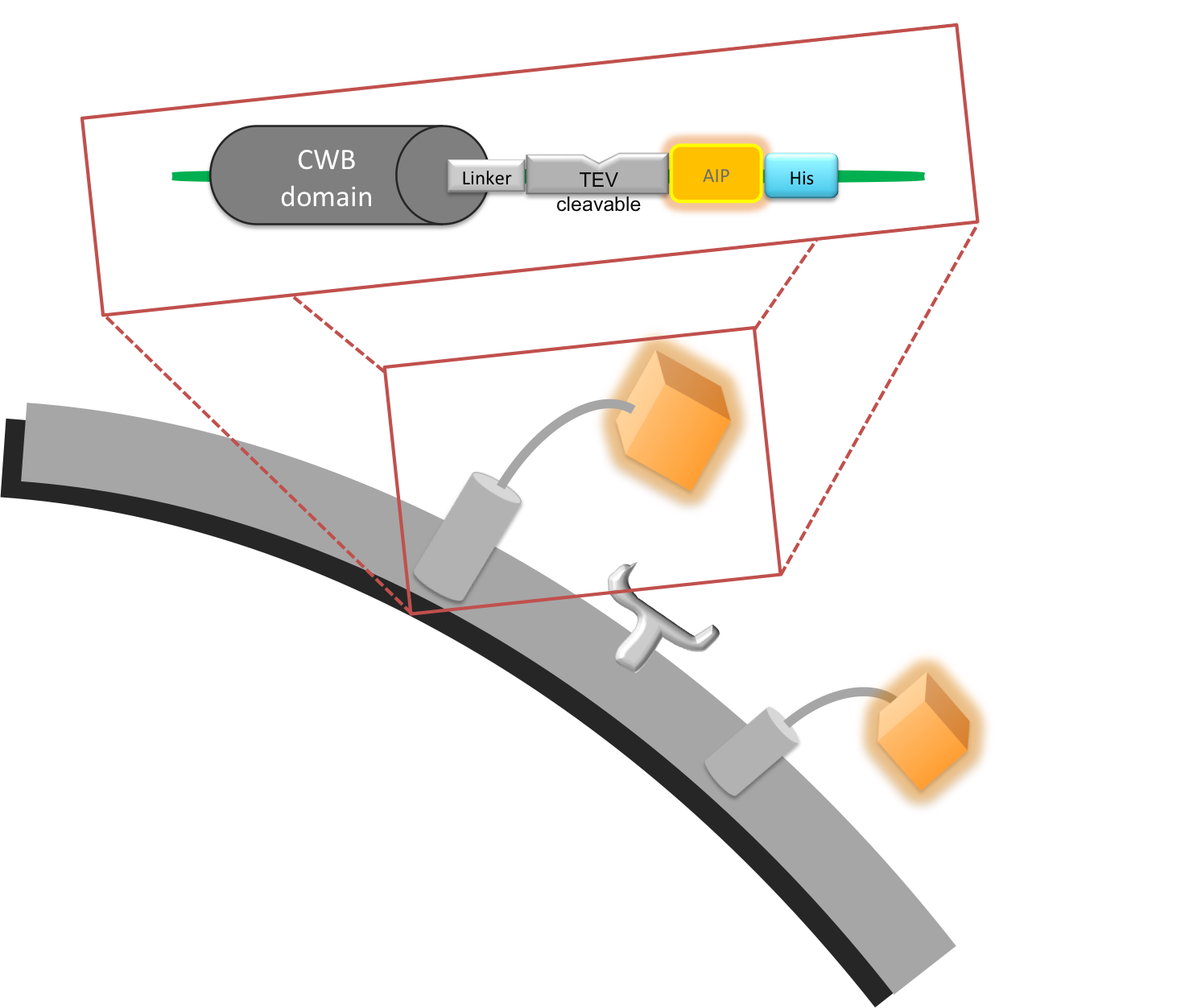
Introduction: This part was used to link the cell wall binding domain (CWB) of LytC BBa_K316030, with the quorum sensing peptide (AIP) as well as providing a cleavage site for a protease we want to detect. This construct was built as a protease detection unit.
LytC:
The part carries part of LytC on its 5’ end. This was used to ligate the linker with LytC via an internal AccI restriction site that occurs naturally in B. subtilis LytC sequence.
Glycine Linker:
The Linker separates the CWB and the AIP and creates space for the protease to access the cleavage site; it consists of two main sections. The first six amino acids (SRGSRA) were suggested to be used specifically with LytC1 . The second section consists of a several glycine residues.
Elastase Cleavage Site:
This sequence forms the 3’ end of the linker and is directly attached to the 5’ end of the AIP. It is four amino acids (SWPL) long and was designed to be efficiently cleaved by the schistosoma cercarial elastase.
His-Tag:
To be able to purify the protein for testing, we attached a His-Tag on our linker-AIP peptide. As it would probably interfere with recognition of the AIP by the receptor it has to be removed from the final construct.
Stop Codon:
In order to end translation a double stop codon was put in place.
For more information about this part of our project please visit our wiki [http://2010.igem.org/Team:Imperial_College_London/Strategy] or take the tour [http://2010.igem.org/Team:Imperial_College_London/Tour/Page_One] to learn more about the project.
Figure I. Graphical representation of cell wall binding protein.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
<biblio>
- 1 pmid=14594841
</biblio>
| None |