Part:BBa_K2298002
Homo sapiens platelet derived growth factor subunit A (PDGFA)
Usage and Biology
Platelet-derived growth factors family consists of five members of homodimeric or heterodimeric protein. By binding to PDGFR-alpha or PDGFR-beta, two tyrosine kinase receptors, PDGFs trigger signaling cascades that regulate cellular behaviors, including chemotaxis, cell proliferation, survival and differentiation. It is believed that PDGFs play essential roles in all stages of wound healing.【1】
Prior studies show that adding Platelet-derived growth factor (PDGF) directly to experimentally designed wounds enhances wound healing【2】.
Design Considerations
The nucleotide sequence of PDGF-A mRNA was retrieved from NCBI nucleotide database(NCBI Reference Sequence NM_033023.4:844-1434), and amplified from human cell cDNA with PCR. Biobrick prefix and suffix was added by PCR using the following primers,
PDGFA prefix: 5’ CGGAATTCGCGGCCGCTTCTAGATGAGGACCTTGGCTTGCCT 3’
PDGFA suffix: 5’ AACTGCAGCGGCCGCTACTAGTACCTCACATCCGTGTCCTCTTCC 3’
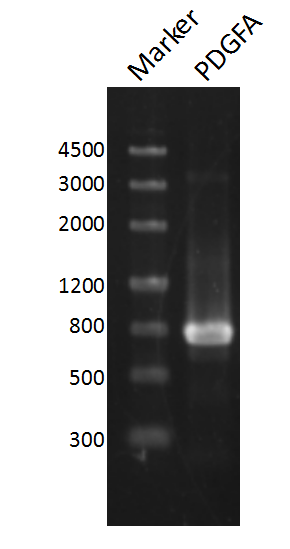
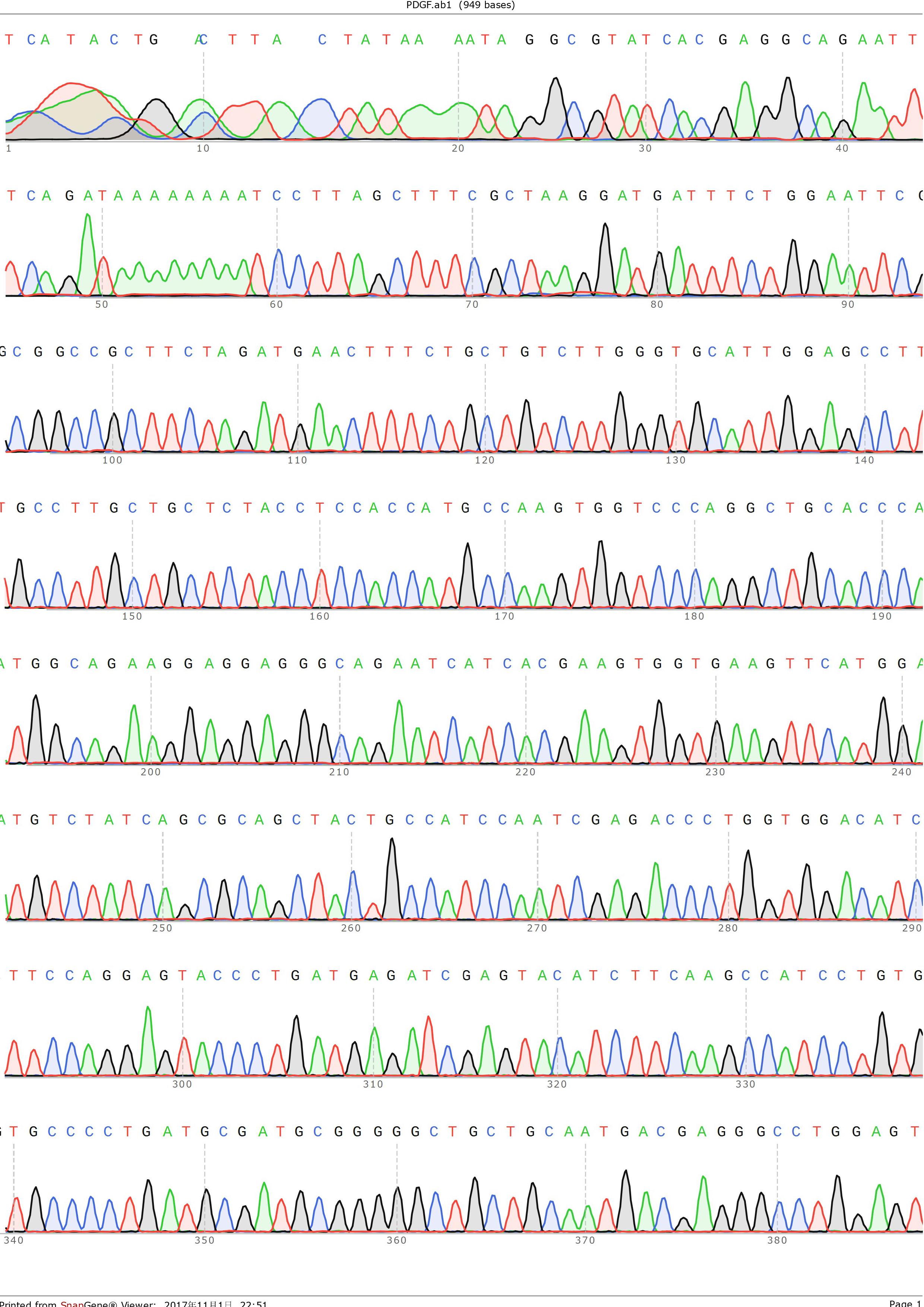
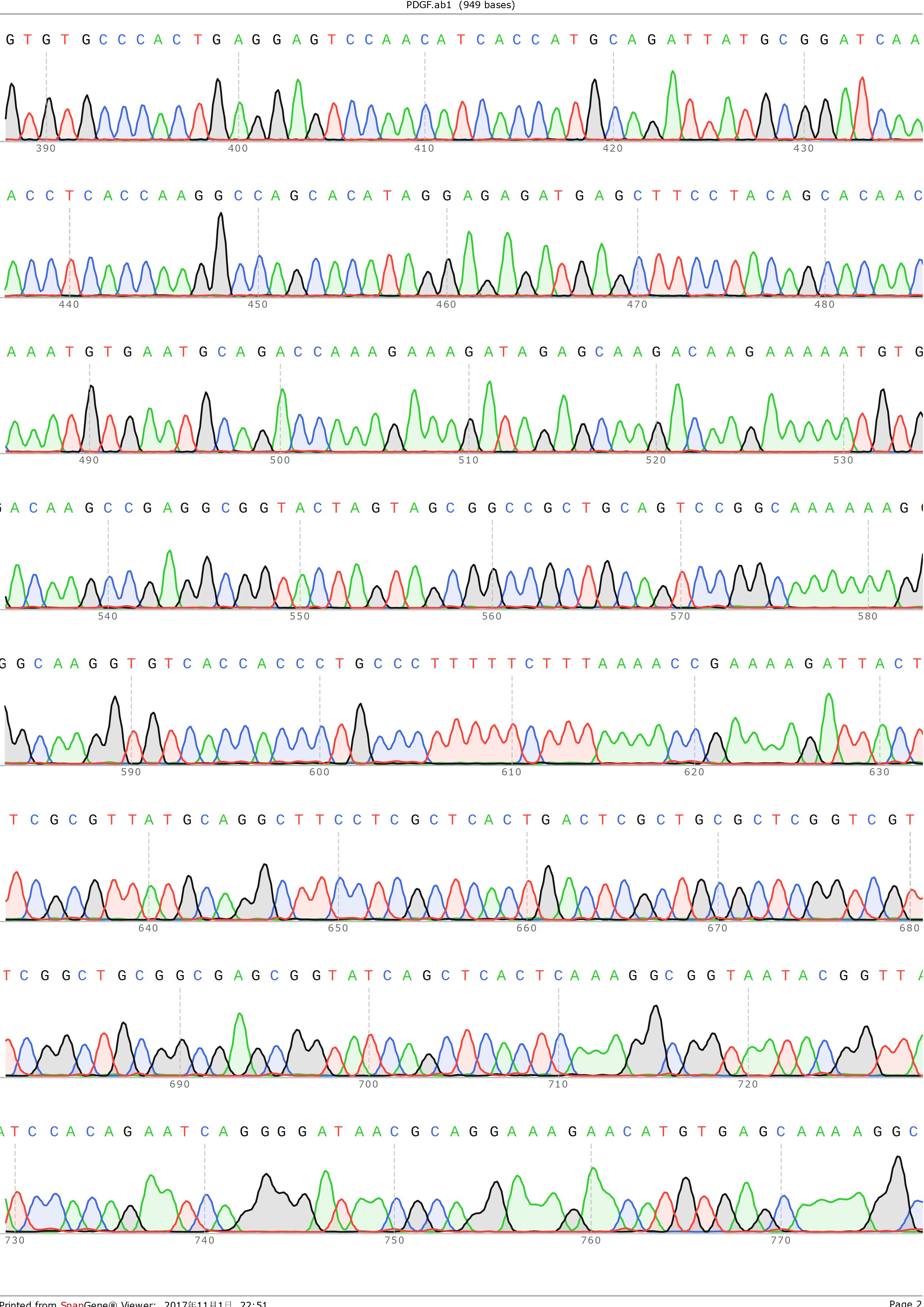
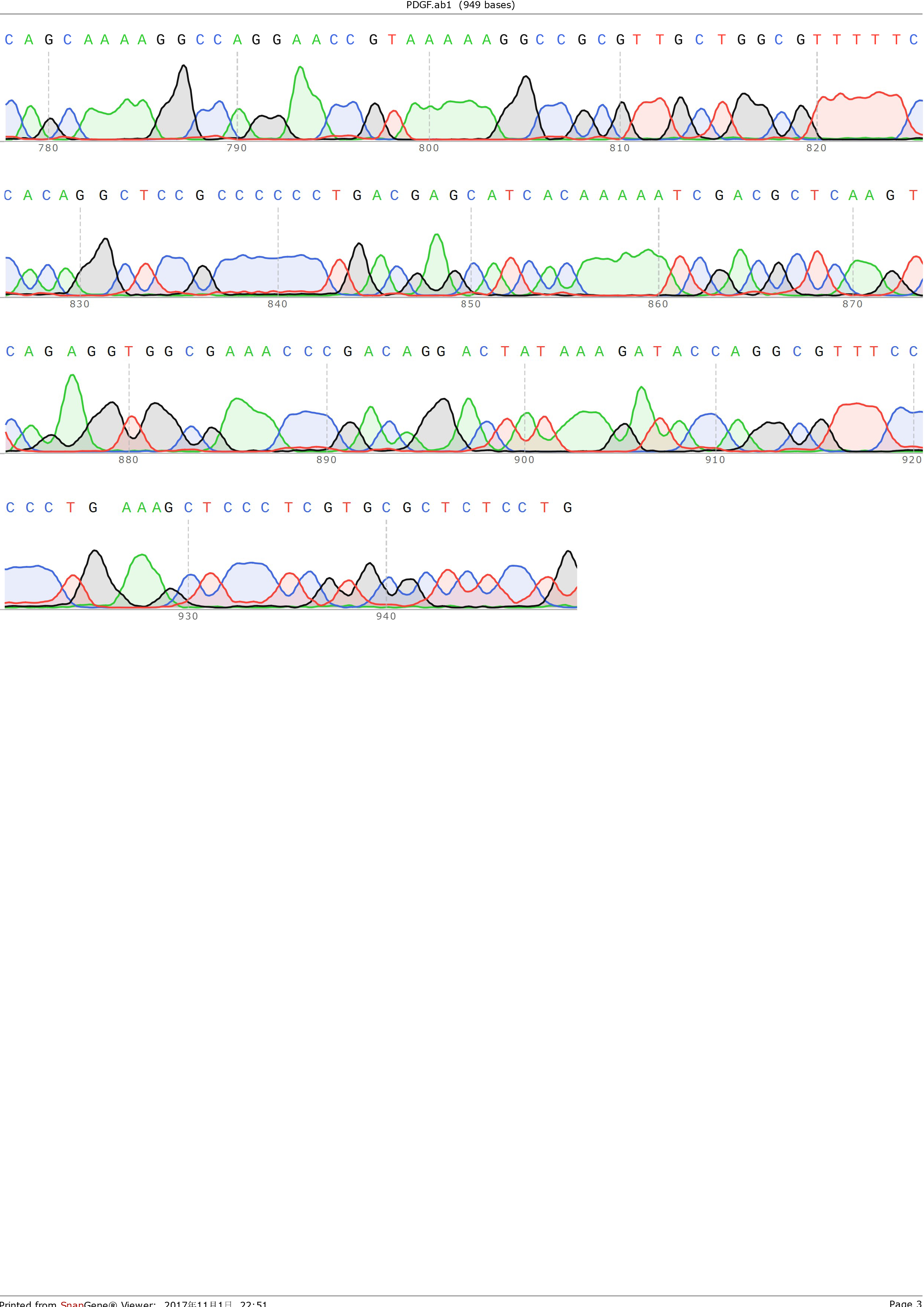
and ligated onto the pSB1C3 plasmid backbone obtained from digestion of InterLab test device 1(Part:BBa_J364000). The ligation was verified by PCR using VF2 and VR as primers, and was further comfirmed by Sanger sequencing by IGE Biological LTD. using VF2 as the forward primer.
Figure 3: Sequencing result of pSB1C3-PDGFA, using VF2 as primer
Note that this part comes in the absence of the stop codon at the end of the sequence, hence this part should be cloned into vectors with pre-existing stop codon, fused with other protein with stop codon, or added a stop codon using PCR prior to use.
2-step PCR
When it comes to constructing BioBricks, it is essential to add both BioBrick prefix and suffix However, both prefix and suffix is 22bp in length with high GC content, resulting in primers over 40bp in length, accompanied by extremely high Tm value. PCR reactions with such primers are likely to yield no intended products. Last year, the team SYSU-CHINA proposed to use shorter primers with only XbaI and SpeI restriction sites to solve this problem. However, XbaI and SpeI have compatible sticky ends which may result in uncontrolable orientation of the insertion as well as vector self-ligation (unless treated with alkaline phosphatase).
This year, we propose an alternative PCR protocol called 2-step PCR (there are only 2 steps each cycle) to solve this problem. This method is adapted from Takara PrimerSTAR Max DNA Polymerase product manual with slight alternation. The set-up is shown below:
For the reaction mixture:
DNA template: 200-300ng/50ul
Forward primer: 20pmol
Reverse primer: 20pmol
PrimeSTAR Max Premix(2×): 25ul
DdH2O: up to 50ul
For the reaction condition set-up:
Pre-denaturalization
98 ℃ for 3minutes
Amplification cycles
95 ℃ for 30 seconds
68 ℃ for 60 seconds
Repeat for 30 cycles
Final elongation
72 ℃ for 3 minutes
Using this method, we successfully added prefix and suffix to the VEGF-A121 gene and ligate the product to pSB1C3 backbone with ease.
Note that this method may yield unspecific amplification, thus agarose gel electrophoresis and gel extraction should be performed to obtain amplification products of the right size. Moreover, additional experiments are required to determine the optimal parameters for this PCR reaction.
Results
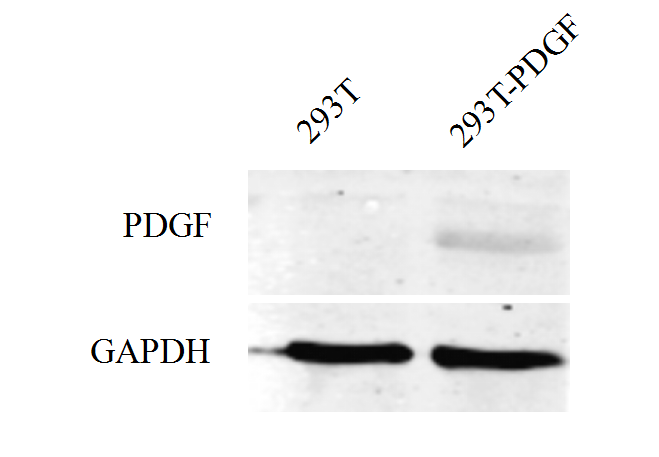
In iGEM 2017, SYSU-CHINA constructed the circuit in which PDGFA was fused to eGFP as well as 3xFLAG tags and expressed under the control of CMV promoter. In addition, SYSU-CHINA teamed up with SCUT-CHINA_A to perform an expression experiment. HEK293T was transfected with plasmid containing the pCMV-PDGFA-eGFP-3xFLAG device, and the expression was detected using Western Blot.
For more details, please check out SCUT-CHINA_A's [http://2017.igem.org/Team:SCUT-China_A/Practice_Collabaration collabaration page]!
References
1 Kaltalioglu, K. & Coskun-Cevher, S. A bioactive molecule in a complex wound healing process: platelet-derived growth factor. International journal of dermatology 54, 972-977, doi:10.1111/ijd.12731 (2015).
2 Ross, R., Bowen-Pope, D. F. & Raines, E. W. Platelet-derived growth factor and its role in health and disease. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 327, 155-169 (1990).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |