Part:BBa_K2298001
vascular endothelial growth factor A (VEGFA)-121
Biology and Usage
Vascular endothelial growth factor (VEGF) represents a family of homodimer glycoproteins which plays a critical role for vasculogenesis, lymphangiogenesis as well as angiogenesis. In humans, VEGF-A exists in six different isoforms, VEGF-A121, VEGF-A145, VEGF-A165, VEGF-A183, VEGF-A189 and VEGF-A206, as a result of alternative splicing of the precursor mRNA. VEGF-A121, the smallest variant, diffuses freely due to the lack of heparin binding site while others interact with extracellular matrix and cell surface heparan sulphate proteoglycans【1】.
VEGFs stimulate cellular responses through binding to cell surface receptor tyrosine kinases, namely VEGFR-1, VEGFR-2 and VEGFR-3, therefore triggering downstream signaling cascades. The responses include endothelial cell proliferation, migration, survival and vascular permeability alternation【1】. In addition, VEGFs stimulate keratinocytes and fibroblasts to migrate towards the wounded site, which also contributes to wound healing【2】.
In a study on VEGFs’ effect on endometrial repair in severe intrauterine adhesion, VEGF expression and micro-vessel density(MVD) in patients responding to therapy were significantly higher than those in patients non-responding to therapy, indicating a critical role of VEGF in the healing process of endometrial after surgery【3】.
Improvement on a previous part
In iGEM 2012, the team Slovenia submitted a composite part Part:BBa_K782061 containing VEGF, p2A sequence and PDGF. We decided to improve the part by submitting a basic part version of VEGF-A121 for teams in the future, so that they can use this growth factor alone or in combination with other growth factors other than PDGF. In addition, the VEGF coding sequence from the original part was from mouse(Mus musculus). So our new part improve it by submitting the counterpart in human(Homo sapiens), which we believed is more appropriate for experiments using human cell lines as well as clinical application.
Design Considerations
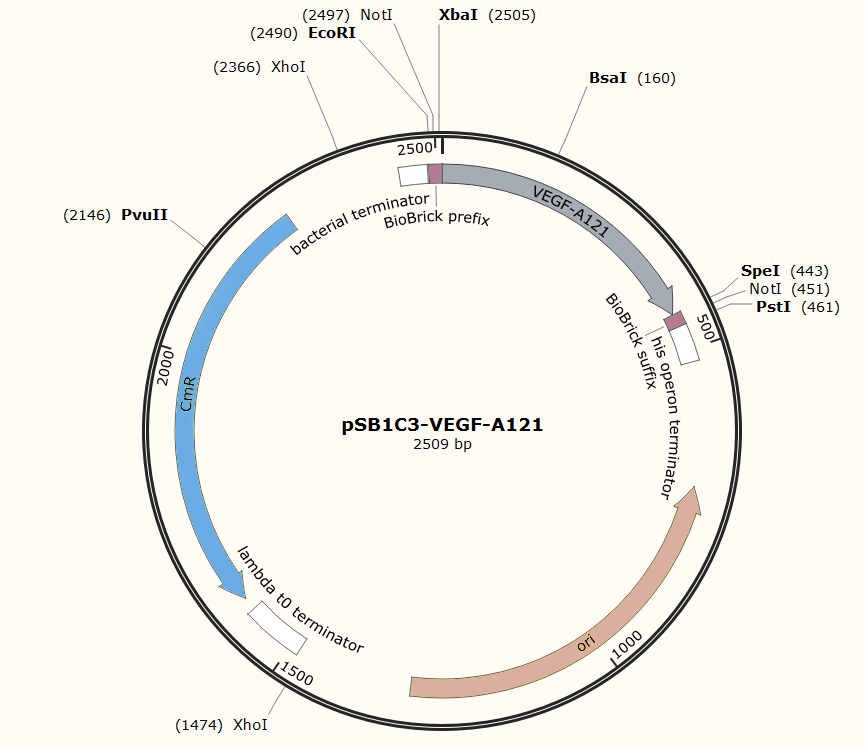
The Brick The nucleotide sequence of VEGFA-121 mRNA was retrieved from NCBI nucleotide database(NCBI Reference Sequence: NM_001025370.2: 1039-1479), and synthesized by IGE Biotechnology LTD.. Biobrick prefix and suffix was added by PCR using the following primers,
VEGF-A121 prefix: 5’ CGGAATTCGCGGCCGCTTCTAGATGAACTTTCTGCTGTCTTGGGT 3’
VEGF-A121 suffix: 5’ AACTGCAGCGGCCGCTACTAGTACCGCCTCGGCTTGTCAC 3’
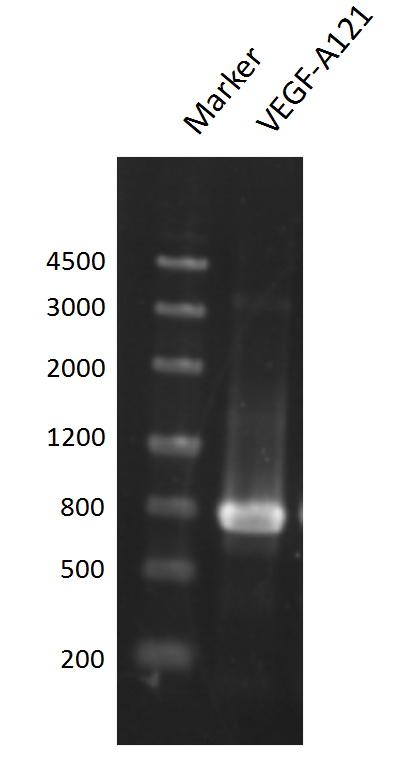
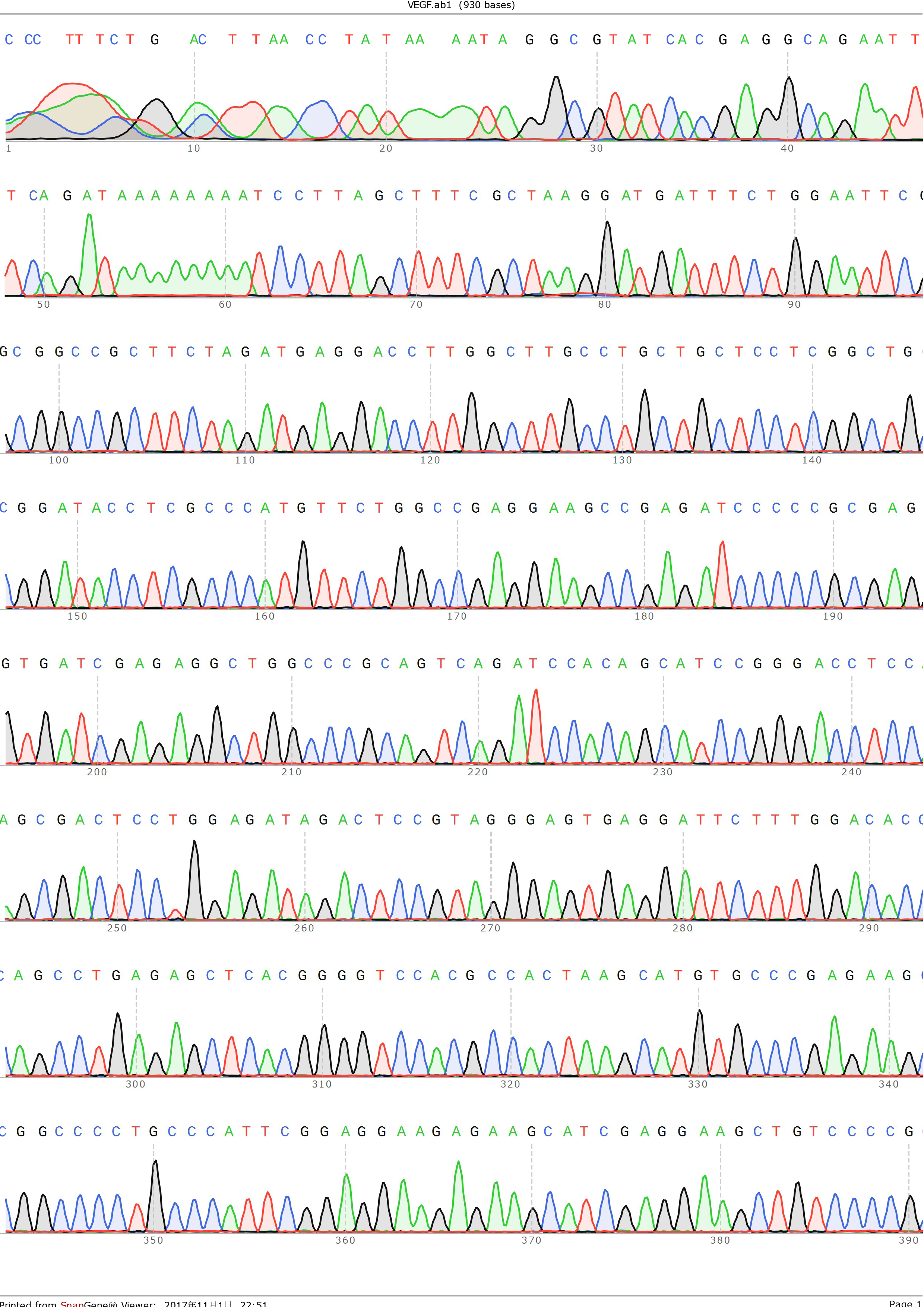
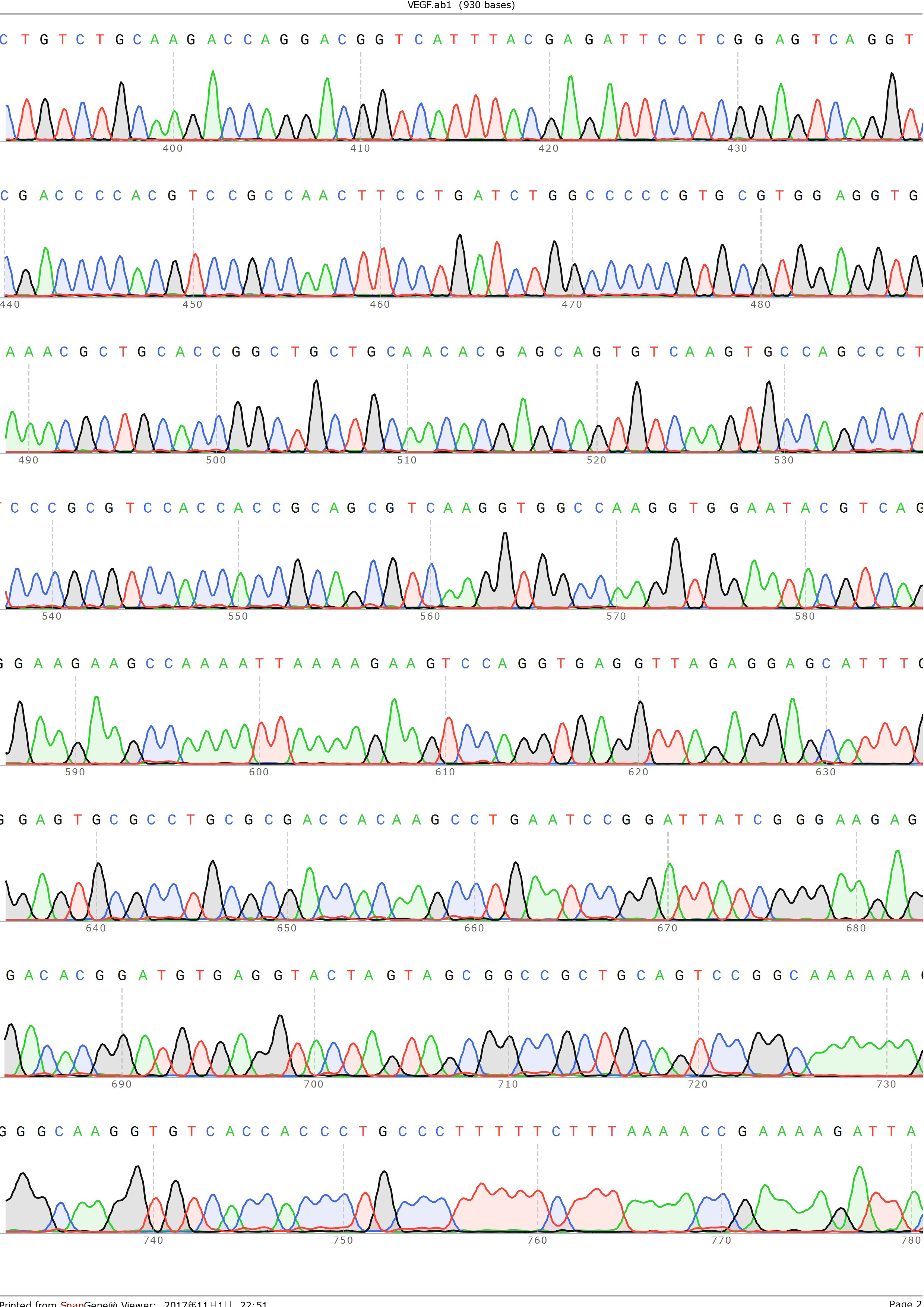
and ligated onto the pSB1C3 plasmid backbone obtained from digestion of InterLab test device 1(Part:BBa_J364000). The ligation was verified by PCR using VF2 and VR as primers, and was further comfirmed by Sanger sequencing by IGE Biotechnology LTD using VF2 as the forward primer.
Figure 4: Sequencing result of pSB1C3-VEGF-A121, using VF2 as primer
Note that this part comes in the absence of the stop codon at the end of the sequence, hence this part should be cloned into vectors with pre-existing stop codon, fused with other protein with stop codon, or added a stop codon using PCR prior to use.
2-step PCR
When it comes to constructing BioBricks, it is essential to add both BioBrick prefix and suffix However, both prefix and suffix is 22bp in length with high GC content, resulting in primers over 40bp in length, accompanied by extremely high Tm value. PCR reactions with such primers are likely to yield no intended products. Last year, the team SYSU-CHINA proposed to use shorter primers with only XbaI and SpeI restriction sites to solve this problem. However, XbaI and SpeI have compatible sticky ends which may result in uncontrolable orientation of the insertion as well as vector self-ligation (unless treated with alkaline phosphatase).
This year, we propose an alternative PCR protocol called 2-step PCR (there are only 2 steps each cycle) to solve this problem. This method is adapted from Takara PrimerSTAR Max DNA Polymerase product manual. The set-up is shown below:
For the reaction mixture:
DNA template: 200-300ng/50ul
Forward primer: 20pmol
Reverse primer: 20pmol
PrimeSTAR Max Premix(2×): 25ul
DdH2O: to a total volume of 50ul
For the reaction condition set-up:
Pre-denaturalization
98 ℃ for 3minutes
Amplification cycles
95 ℃ for 30 seconds
68 ℃ for 60 seconds
Repeat for 30 cycles
Final elongation
72 ℃ for 3 minutes
Using this method, we successfully added prefix and suffix to the VEGF-A121 gene and ligate the product to pSB1C3 backbone with ease.
Note that this method may yield unspecific amplification, thus agarose gel electrophoresis and gel extraction should be performed to obtain amplification products of right size. Moreover, additional experiments are required to determine the optimal parameters for this PCR reaction.
Results
For more details, please check out our [http://2017.igem.org/Team:SYSU-CHINA#/Demonstrate result page]!
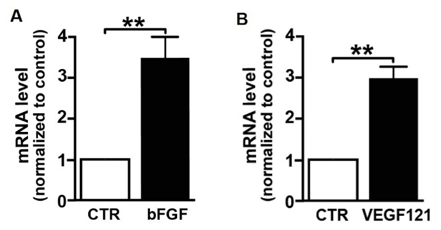
In iGEM 2017, SYSU-CHINA constructed the circuit in which VEGF-A121 was fused to eGFP tags as well as 3xFLAG and expressed under the control of CMV promoter. Bone marrow stromal cells (BMSCs) were infected the with lentivirus containing the circuit and selected using puromycin. The transcription of VEGF-A121 was measured using quantative PCR.
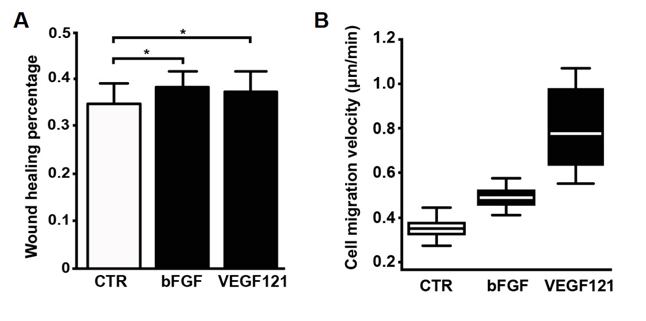
The VEGF-A121-transgenic BMSCs was cultured for 48 hours and the culture supernatant, theoratically containing certain growth factors, was collected. Scratch wound healing assays were performed using HEK293T cells with the collected supernatant. The data below suggested that VEGF-A121 produced by transgenic BMSCs is able to accelerate wound healing.
To further demonstrate the potentials of the VEGF-A121-transgenic BMSCs, Collected supernatant mentioned above was used to culture uterine endometrium cells, and MTT assay were performed after 48 hours. The result indicated that VEGF-A121 secreted by engineered BMSCs is able to promote endometrium cell growth.
References
1 Holmes, K., Roberts, O. L., Thomas, A. M. & Cross, M. J. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cellular signalling 19, 2003-2012, doi:10.1016/j.cellsig.2007.05.013 (2007).
2 Bao, P. et al. The role of vascular endothelial growth factor in wound healing. The Journal of surgical research 153, 347-358, doi:10.1016/j.jss.2008.04.023 (2009).
3 Chen, Y., Chang, Y. & Yao, S. Role of angiogenesis in endometrial repair of patients with severe intrauterine adhesion. International journal of clinical and experimental pathology 6, 1343-1350 (2013).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 166
| None |