Part:BBa_K2273017
| Part Information | |
|---|---|
| RFC standard | RFC 25 |
| Partner Tag | BBa_K2273014: SpyTag |
| Original Biobrick Part | BBa_K1159200: SpyCatcher |
| Non-tagged mini.SpyCatcher | BBa_K2273015: mini.SpyCatcher |
| Submitted by | [http://2017.igem.org/Team:TU_Dresden TU Dresden] |
Minimal SpyCatcher codon optimized for Bacillus subtilis with His Tag
This is a shortened and codon adapted version of the SpyCatcher improved for secretion in B. subtilis. It codes for a part of the fibronectin-binding protein FbaB from Streptococcus pyogenes that forms a covalent isopeptide bond with a short oligopeptide called SpyTag. There is the original (BBa_K1159201) and the codon adapted version (BBa_K2273014) of this oligopeptide in the registry. The SpyTag and the Minimal SpyCatcher can be fused as a Tag to proteins of interest and will then mediate covalent bonding of these proteins.
Improvement
Minimization:
The SpyCatcher is a highly functional Tag, that can interact with its protein partner: the SpyTag. Upon interaction, additional C- or N-terminally translational fused proteins are brought in close proximity and can be linked together via this Tag system. Unfortunately the size of the SypCather (116 amino acid) is quite large and can therefor hinder the native folding of a linked the protein of interest.The 2017 team of [http://2017.igem.org/Team:TU_Dresden TU_Dresden] submitted a minimized version of the SpyCatcher to the registry to ensure the effective [http://2017.igem.org/Team:TU_Dresden/Project/Secretion secretion] necessary for their project. The mini. SpyCatcher is 32 amino acids shorter but still fully functional, which was proven by TU_Dresden team (see Application) as well as by the researchersthat proposed the minimization.
Codon adaption:
The original part was used in E. coli but the mini. SpyCatcher was thought to be implemented throughout TU_Dresdens project in B. subtilis. Therefore the sequence was codon adapted for this purposes. The sequence was proposed by researchersthat investigated the secretion of SpyTag/SpyCatcher system in B. subtilis.
His-Tag:
The mini. SpyCatcher was submitted to the registry with an added C-terminal His-Tag to purify the protein constructs when SpyTag/SpyCatcher link together.
Application
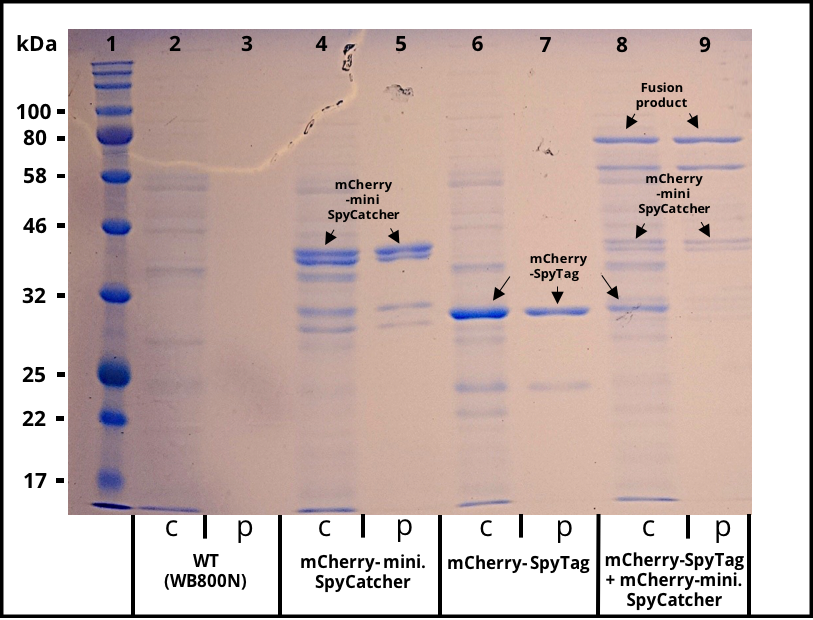
The 2017 team of [http://2017.igem.org/Team:TU_Dresden/Project/Secretion TU_Dresden] used mini. SpyCatcher together with its partner SpyTag to investigate the potential of using B. subtilis secretion system for the production of self conjugation multi protein complexes. The his-tagged mini. SpyCatcher was C-terminally fused to mCherry which functions as a reporter protein. The secretion was mediated through the N-terminal signal peptide of AmyE. The secretion of the functional mCherry fusion protein could be proven (Figure 1 and Figure 2). The functionality of the mini. SpyCatcher was proven through SDS-Page (Figure 3). After incubating supernatants containing mCherry-mini. SpyCatcher and mCherry-SpyTag the fusion product was detected, proving the covalent bonding of the Tag partners.

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |

