Part:BBa_R0071
Promoter (RhlR & C4-HSL regulated)
Promoter activated by RhlR in concert with C4-HSL. C4-HSL is produced by RhlI, and acts as a quorum-sensing autoinducer.
This is the natural sequence taken from Pseudomonas aeruginosa.
Crosstalk: this promoter is also activated at a low level by LasR with its associated HSL.
Usage and Biology
RhlR binds to the promoter region and activates transcription when in the presence of its autoinducer -- N-butyryl-homoserine lactone (also known as C4-HSL), which is produced by RhlI.
From [Medina03]: <i>... RhlR binds to this DNA sequence, both in vivo and in vitro, in the presence and in the absence of C4-HSL. The binding of RhlR(C4-HSL) to the las box is a necessary condition for rhlAB expression while the binding of RhlR alone to the same DNA sequence seems to repress rhlAB transcription.</i>
From [Medina03]: <i>The LasR(3-O-C12-HSL) complex can activate rhlAB promoter expression but at a considerably lower level than the RhlR(C4-HSL) complex.</i>
From the data in [Medina03], LasR activates rhlAB at 7.8% of the activation level achieved by RhlR.
From the data in [Pearson97], the activation by LasR is about 3% of that by RhlR.
Improvement and Characterization
Group: Tokyo Tech 2016
Author: Yoshio Takata
Summary of Improvement and Characterization:
I. Improved Prhl by iGEM 2014 Tokyo_Tech team and characterize RhlR assay
II. Improvement of the wild type Prhl
III. Comparison of the improved Prhl by iGEM 2014 Tokyo_Tech to our original improved Prhl
We Tokyo Tech 2016 simulated our final genetic circuits and found that the circuits did not work, because Prhl strength was too weak. (see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]). We therefore considered using the improved Prhl (BBa_K1529310,BBa_K1529310) established by Tokyo_Tech 2014, but we noticed that they were inappropriate for two reasons (see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]). Then, we decided to improve Prhl Promoter and obtain our original improved Prhl (included in BBa_K1949060) that suited our goal.
Our purpose is to create strong Prhl for our final genetic circuits. This experiment consists of the three parts below.
I. Improved Prhl by iGEM 2014 Tokyo_Tech team and characterize rhlR assay
II. Improvement of the native Prhl
III. Comparison of the improved Prhl by iGEM 2014 Tokyo_Tech team to our original improved Prhl
The past improved Prhl did not suit for our final circuits and we could create the improved Prhl appropriate to our final circuits.
I. Improved Prhl by iGEM 2014 Tokyo_Tech item and characterize RhlR assay
We found that Prhl(RL) (BBa_K1529300) activity was weak and the expression level depended on LVA tag (Fig.1); LVA-tagged proteins are prone to be degraded by cellular proteases. Prhl(LR) (BBa_K1529310) activity was strong and unexpectedly reacted with C12 (crosstalk) (Fig.3).
The colonies of transformants with a rhlR (BBa_C0171) plasmid looked rough and the growth rate was low(Fig.2-left), while the colonies of transformants with a rhlR-LVA (BBa_C0071) plasmid looked smooth and the growth rate was normal(Fig.2-right). However, the reason for this result is unclear.
II. Improvement the wild type Prhl
By introducing a single point mutation into wild type Prhl (BBa_R0071) by PCR, we obtained 198 Prhl mutants and chose the two Prhl mutants of which promoter activity was stronger than wild type Prhl(Fig.3).
The sequences of these two chosen mutants are shown below. The small characters indicate the scar sequence and a single point mutation is colored with red.
Native Prhl (BBa_R0071)
TCCTGTGAAATCTGGCAGTTACCGTTAGCTTTCGAATTGGCTAAAAAGTGTTC
Prhl(NM)(BBa_K1949060)
TCCTGTGAAATCTGGCAGTTACCGTTAGCTTTCGAATTGGCTAtAAAGTGTTC
III. Comparison the improved Prhl by iGEM 2014 Tokyo_Tech team to our original improved Prhl
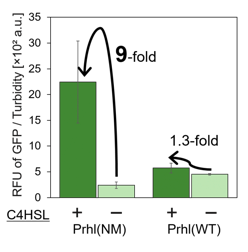
Prhl(NM) was chosen from the many Prhl mutants, and by comparing Prhl(NM) to Prhl(LR), we obtained the result below (Fig.4). The reaction activity of Prhl(NM) to C4 was stronger than that of Prhl(LR), and Prhl(NM) did not react with C12 at all.
If you want more information, you see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]!
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Functional Parameters
| biology | -NA- |
| direction | Forward |
| negative_regulators | -NA- |
| positive_regulators | 1 |
Functional Parameters: Austin_UTexas
Burden Imposed by this Part:

Burden is the percent reduction in the growth rate of E. coli cells transformed with a plasmid containing this BioBrick (± values are 95% confidence limits). This BioBrick did not exhibit a burden that was significantly greater than zero (i.e., it appears to have little to no impact on growth). Therefore, users can depend on this part to remain stable for many bacterial cell divisions and in large culture volumes. Refer to any one of the BBa_K3174002 - BBa_K3174007 pages for more information on the methods, an explanation of the sources of burden, and other conclusions from a large-scale measurement project conducted by the 2019 Austin_UTexas team.
This functional parameter was added by the 2020 Austin_UTexas team.
//direction/forward
//chassis/prokaryote/ecoli
//promoter
//regulation/positive
//classic/regulatory/uncategorized
//function/cellsignalling/RhlR
| biology | |
| direction | Forward |
| negative_regulators | |
| positive_regulators | 1 |

 1 Registry Star
1 Registry Star