Project
Part:BBa_K404254
Designed by: Freiburg Bioware 2010 Group: iGEM10_Freiburg_Bioware (2010-10-14)
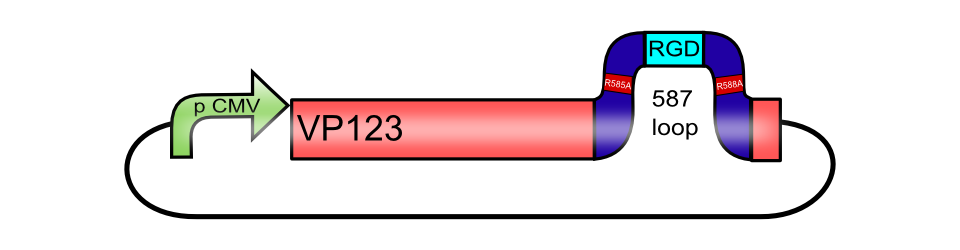
pCMV_[AAV2]-VP123ex (ViralBrick-587KO-RGD)
The RGD integrin binding motif including a knockout of the natural HSPG tropism BBa_K404212, inserted into the 587 loop of pCMV_[AAV2]-VP123
| [pCMV_[AAV2-VP123ex(ViralBrick-587KO-RGD] | |
|---|---|
| BioBrick Nr. | BBa_K404254 |
| RFC standard | RFC 10 |
| Requirement | pSB1C3 |
| Source | pAAV_MCS provided by Stratagene |
| Submitted by | [http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] |
Usage and Biology
ViralBrick-587KO-RGD
Integrins are transmembrane proteins that, among other functions, mediate cell attachment to surrounding tissues. They bind to a motif consisting of the amino acids arginine, glycine and aspartic acid (RGD in one-letter code). Because Integrin is highly expressed in many tumor cell lines (Albelda et al., 1990), (Damjanovich, Albelda, Mette, & Buck, 1992), (Lessey et al., 1995), (Smythe, LeBel, Bavaria, Kaiser, & Albelda, 1995), (Gladson & Cheresh, 1991), AAV particles displaying the RGD motif on various positions in their capsid proteins have been created by (Shi et al., 2003). Particles displaying RGD at amino acid positions 584 & 588 as well as 453 or 587 (Boucas et al., 2009) showed transduction efficiencies similar to wt AAV, even when the cells’ HSPG receptors were blocked by heparin sulfate or when the natural HSPG binding motif on the capsid surface was knocked out. To further broaden the area of therapeutic application, we created a ViralBrick containing the RGD motive to specifically target cells with low HSPG-/high Integrin expression.
[AAV2]-VP123ex
The pSB1C3_001_CMV_VP123 contains a CMV promoter upstream the VP123 sequence. The AAV capsid consists of 60 capsid protein subunits. The three cap proteins VP1, VP2, and VP3 are encoded in an overlapping reading frame. Arranged in a stoichiometric ratio of 1:1:10, they form an icosahedral symmetry. The mRNA encoding for the cap proteins is transcribed from p40 and alternative spliced to minor and major products. Alternative splicing and translation initiation of VP2 at a nonconventional ACG initiation codon promote the expression of VP1, VP2 and VP3. The VP proteins share a common C terminus and stop codon, but begin with a different start codon. The N termini of VP1 and VP2 play important roles in infection and contain motifs that are highly homologous to the phospholipase A2 (PLA2) domain and nuclear localization signals (BR)(+).
CMV promoter is derived from human Cytomegalovirus, which belongs to Herpesvirus group. All family members share the ability to remain in latent stage in the human body. CMV is located upstream of immediate-early gene. However, CMV promoter is an example of widely used promoters and is present in mammalian expression vectors. The advantage of CMV is the high-level constitutive expression in mostly all human tissues [Fitzsimons et al., 2002].
References
DiPrimio, Asokan, Govindasamy, Agbandje-McKenna, & Samulski, June 2008. Surface loop dynamics in adeno-associated virus capsid assembly. Journal of virology, 167(1), 5178–5189Fitzsimons, H.L., Bland, R.J. & During, M.J. 2002. Promoters and regulatory elements that improve adeno-associated virus transgene expression in the brain. Methods San Diego Calif, 28(2), pp.227-236. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12413421.

Sequence and Features
Assembly Compatibility:
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 2396
Illegal XhoI site found at 698
Illegal XhoI site found at 884 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 665
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 2964
Illegal SapI site found at 1833
[edit]
Categories
Parameters
//chassis/eukaryote/human
//viral_vectors
//viral_vectors/aav
//viral_vectors/aav/capsid_coding
//viral_vectors
//viral_vectors/aav
//viral_vectors/aav/capsid_coding
| None |