Part:BBa_K404201
ViralBrick-453-BAP
| ViralBrick-453-BAP | |
|---|---|
| BioBrick Nr. | BBa_K404201 |
| RFC standard | RFC 25 |
| Requirement | pSB1C3 |
| Source | |
| Submitted by | [http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] |
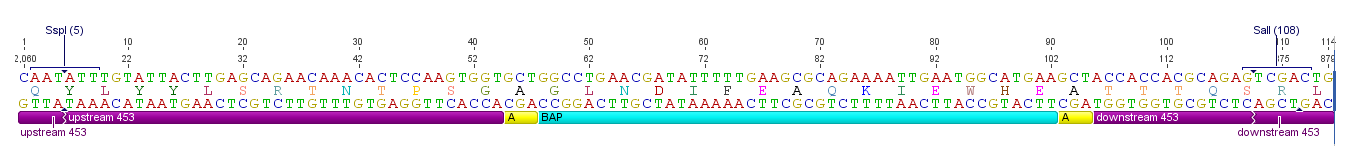
The Biotinylation Acceptor peptide motif, ready for insertion into the 453 loop
| ViralBrick-453-BAP | |
|---|---|
| BioBrick Nr. | BBa_K404201 |
| RFC standard | RFC 25RFC 10 |
| Requirement | backbone without SspI, SalI, BamHI and PvuII |
| Source | synthetic |
| Submitted by | [http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] |
All capsid coding parts (e.g. (AAV2)-RepVP123) of the Virus Construction Kit designed by the iGEM Team Freiburg contain single cutting restriction sites on the side of the sequences coding for the two major surface exposed loops.
Using these restriction sites it is possible to insert functional motifs into the coding sequence for the viral capsid.
This BioBrick contains one of these functional motifs fanked by bilateral linkers and the viral sequences that code for the loop.
This category of BioBricks was termed ViralBrick to clarify that cloning does not function with the usual iGEM RFC restriction sites but with the mentioned ViralBrick restriction sites.
The four restriction sites for the ViralBrick insertion were designed in a way that the amino acid sequence of the viral capsid could be absolutely conserved. This was advisable because even slight changes in the viral capsid lead to drastically changed interactions with cellular surface receptors. For this reason we decided to insert these restriction sites in stead of using BioBrick assembly that would define at least two amino acids in the viral loop.
Restriction sites
Specific Biotinylation via ViralBrick: The Biotinylation Acceptor Peptide
The purified BirA biotin ligase that was kindly provided from Avidity.com
References:

IMAC purification via Viral Brick: The Histidin Affinity Tag
The high binding affinity of Histidine towards metal is being exploited for the purification of proteins via the so called „Immobilized Metal Ion Affinity Chromatography“ (IMAC): Multiple histidine residues (most commonly: Six) are being fused to the end of the targeting protein. A cell extract containing the recombinant protein ist then applied to a collumn containing immobilized Ni2+-Ions. The His-tags covalently bind the Ni-Ions while other cellular proteins can be washed oft he collumn. The purified proteins can then be eluted with Imidazol, which displaces the histidine residues.(Smith et al. 1988), (Hoffmann & Roeder 1991)
Since the aim behind engineering therapeutic AAV vectors is a safe administration to human patients, it is important to consider a convenient way of purifying the virus particles. Contamination by cellular proteins could cause toxic side effects or a strong immune response. Koerber et al. have first inserted a His-tag into a surface-exposed loop at amino acid position 587 in the Cap protein and successfully purified recombinant virsuses using IMAC (Koerber et al. 2007). For our Virus Construction Kit, we provide the His-tag motif in the ViralBrick standard, allowing for an easy insertion into the 453 and/or 587 loop. If the modified capsid bearing a His-tag is being cotransfected with a wild type capsid for the production of mosaic viruses, IMAC helps to not only purify the produced viral particles but also to enrich particles which actually contain the modified proteins.
References:

Targetingpeptides via ViralBrick: The RGD Motif
References:

Coupling Antibodies to the Viral Surface via ViralBrick: The Z34C Motif
This engineered antibody binding domain of 34 amino acids was then inserted into capsids of different viral vectors amongst others also the AAV. In [Ried et al.; 2002] the Z34C domain was inserted at position 587 into the capsid of the AAV resulting in viral vector that can be targeted to different target cells without genetic engineering. This targeting approach was then improved in [Gigout et al.; 2005] by the creation of mosaic vectors that contain only ~25% of recombinant VP-Proteins what resulted in 4 to 5 orders of magnitude more infectiosity compared to all-mutant viruses.
References:


Risk assessment of the Adeno-associated Virus

Please consider special provisions of law in your country before using parts that contribute to the production of genetically modified viral vectors.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
//viral_vectors
//viral_vectors/aav
//viral_vectors/aav/miscellaneous
| None |