Part:BBa_K3215013
Tse2 Repressed by Arabinose Kill Switch
This kill switch was created to enable its user to reduce an organism's growth rate in a medium without arabinose. It is consisted of parts BBa_K259007, BBa_K327018, BBa_B0015, BBa_R0051, BBa_J61100 and BBa_K314200.
Usage and Biology
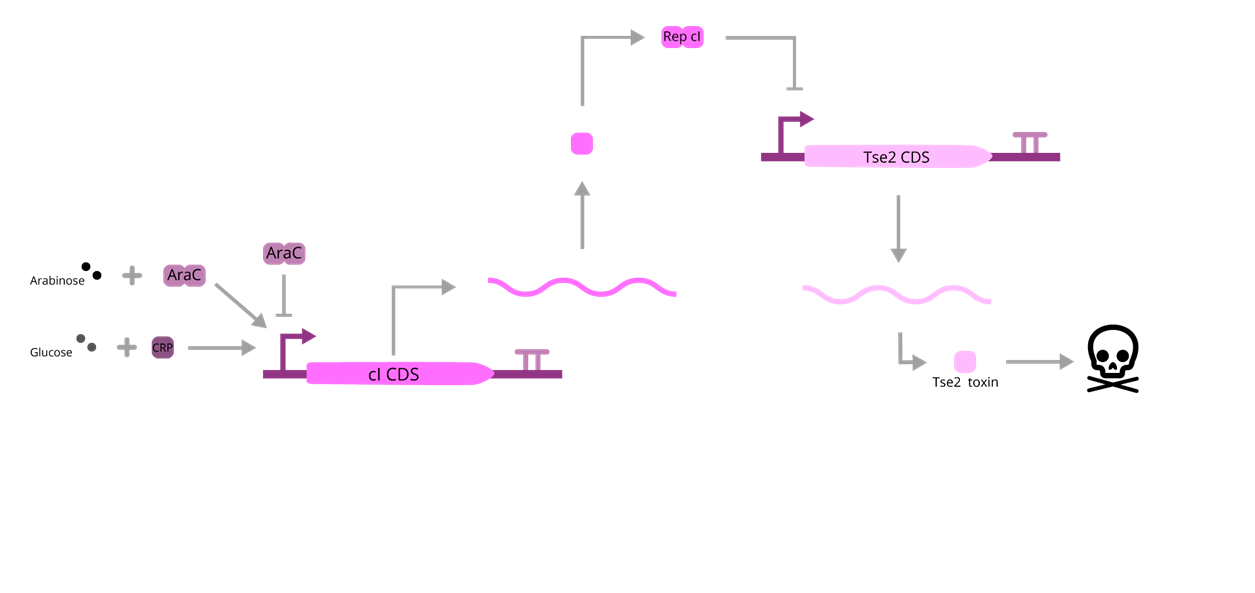
Considering a system where the survival of an organism is inadequate, this BioBrick displays a way of handling the issue. When this mechanism is present, the presence of L-(+)-Arabinose will enhance the transcription of cI, which will repress the expression of the Tse2 toxin, enabling the survival of the organism. On the other hand, when the former is absent, the transctiption of cI will be inhibited, which in turn will activate the expression of Tse2 toxin, repressing the survival rate of those organisms. For our purposes, this explanation lacks one major detail: cAMP. When glucose is absent in the media, the levels of cAMP will rise, and that can disrupt the balance of the system, knowing that cAMP will bind to CRP, enabling the transcription of cI, interfering in the Arabinose regulation.
Fig. 1. BBa_K3215013 scheme.
Characterization
To further characterize and validate our part, two different approaches were made:
Dry lab experiment validation
Our team was able to create a mathematical model that correlates the effects of Arabinose concentration with the levels of cI and Tse2, speculating cell survival and variations of the efficiency of the model in different concentrations of extracellular glucose. To see all the details of our model, please check section 2 from our modelling page, avaiable in Team 2019 UFRGS_Brazil
Results
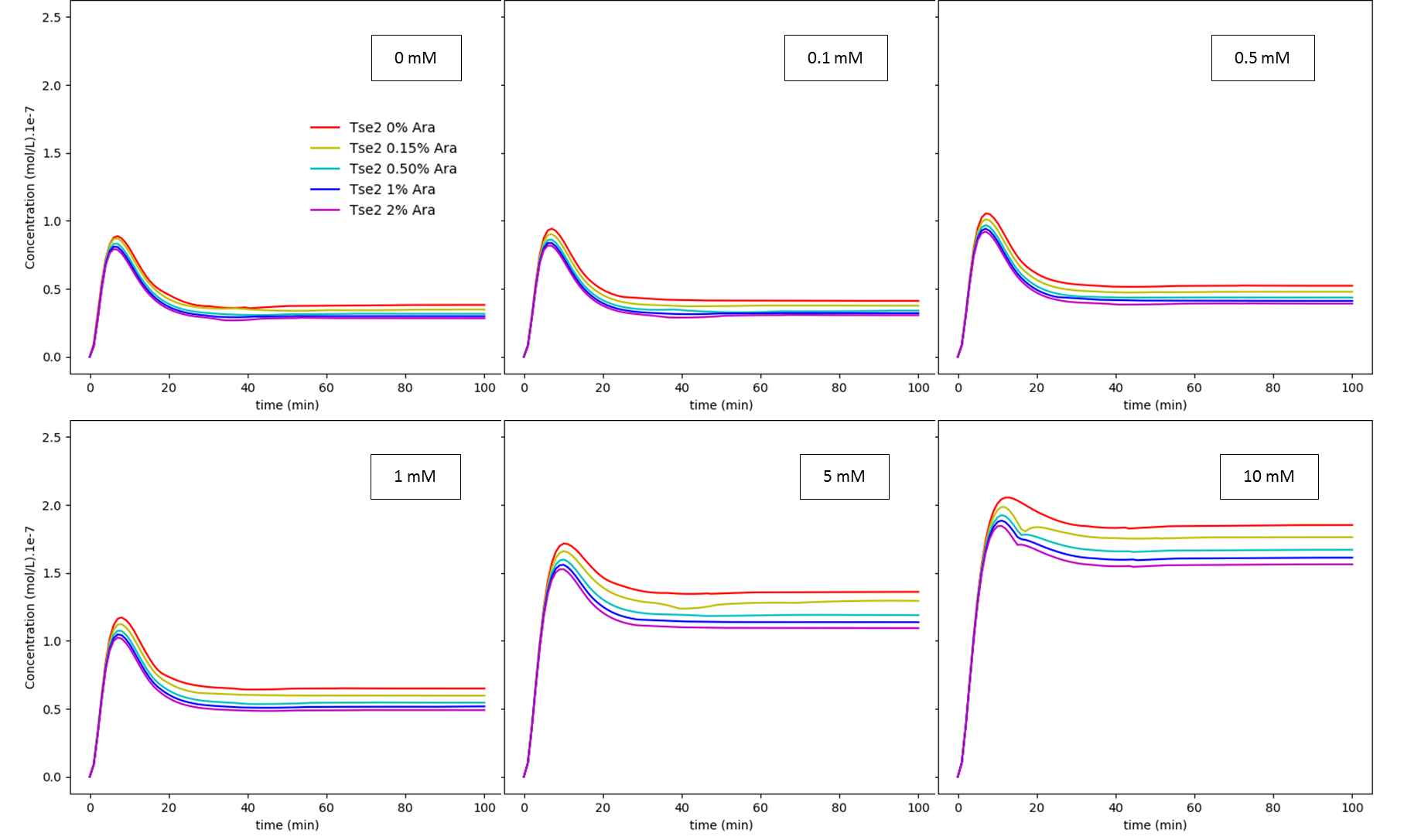
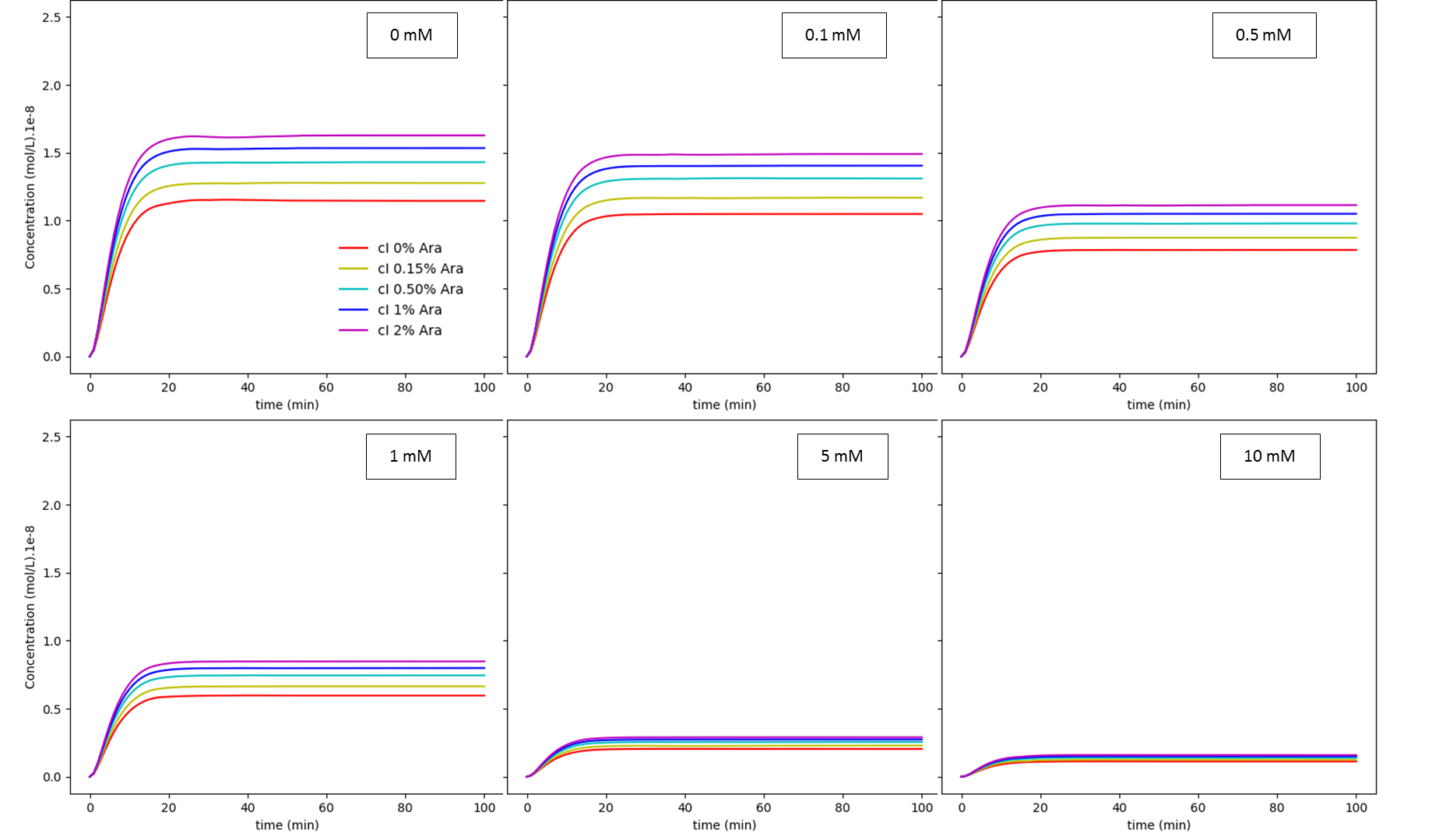
This model operates with 6 variables: (i) arabinose concentration; (ii) glucose concentration; (iii) cI mRNA concentration; (iv) cI concentration; (v) Tse2 mRNA concentration; and (vi) Tse2 concentration. The results are shown below, as a function of concentration of glucose and arabinose, for both cI and Tse2:
Fig. 2. Arabinose regulation dependence on glucose concentration: Tse2. The values shown inside each box are the extracellular glucose concentrations.
Fig. 3. Arabinose regulation dependence on glucose concentration: cI. The values shown inside each box are the extracellular glucose concentrations.
Discussion and conclusions
Like discussed in our modelling page, both results show us the dependence of the system on glucose concentration. When it is subjected to a low glucose concentration, the effect of arabinose becomes much more pronounced. What we can conclude from these experiments is that this system has a very tight cI regulation, which interferes with the rise of Tse2 levels, being difficult to manipulate the concentrations of the intermediates of this biological circuit. Considering all this, we still don’t know if the concentrations of Tse2 for different glucose concentrations, varying arabinose levels, are enough to inhibit bacterial growth or not. Furthermore, more experiments should be done to measure the efficiency of this system. Our model showed that this circuit works, but says almost nothing on how it should work in a living system.
Wet lab experiment validation
Aiming the construction of this part, we ligated a cI Repressor Cassette (AraC promoter fused with RBS + cI CDS + Terminator + cI Repressed promoter) (2) with a Tse2 translation unit fused with a terminator (RBS + CDS + Terminator) (8). Briefly, we digested in an overnight reaction the former with SpeI and EcoRI and the latter with XbaI and PstI. Both were ligated in an overnight reaction with T4 ligase, generating the BBa_K3215013. This new part was inserted into a pSB1K3 plasmid.
To validate this new part, we also developed another new part BBa_K3215012 by fusing the cI Repressor Cassette with a mRFP translation unit fused with a terminator. This part was also inserted into pSB1K3.
All plasmids were inserted into E. coli K-12 by quimioporation.
Results
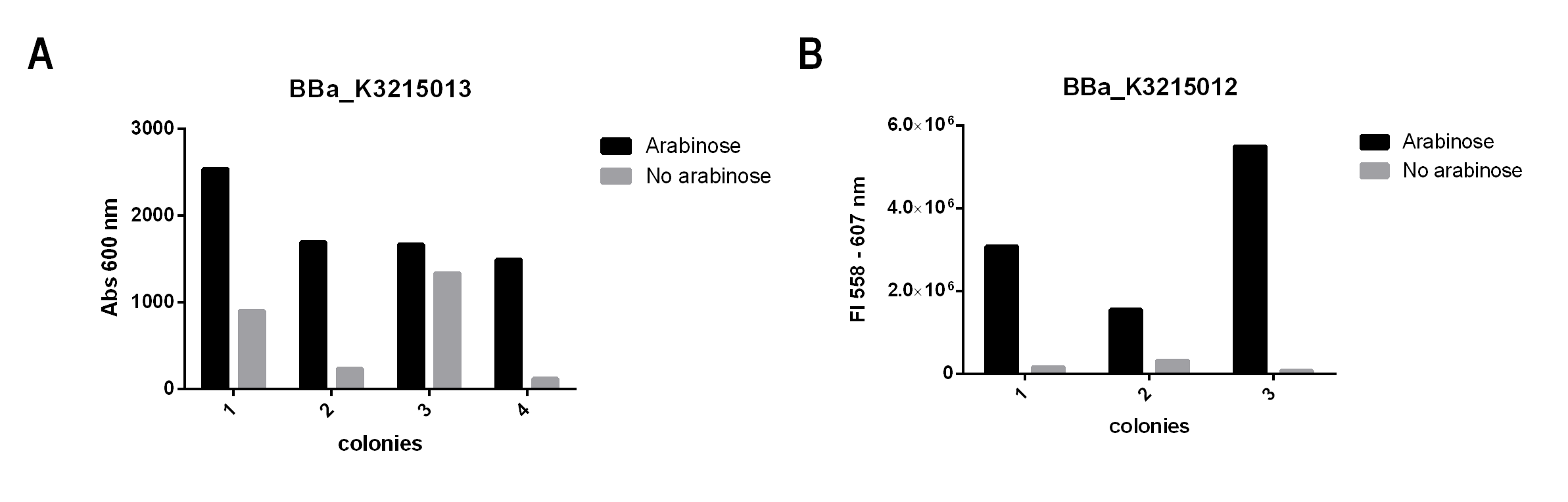
Fig. 4. Biobrick characterization. A) Optical Density (600 nm) of BBa_K3215013 colonies. B) Fluorescence obtained from 558 excitations and 607 emission for BBa_K3215012 colonies.
Discussion and conclusions
As our goal was to create a new biobrick that successfully controls our engineered bacteria growth rate, these data corroborate our biobrick design. As elicited in figure 4. A, bacteria showed bigger growth rates at arabinose supplemented media, as it allows cI to be expressed, controlling the expression of the Tse2 toxin. On the other hand, the colonies growing with no arabinose exhibited high levels of this toxin, which affected its growth. In figure 4. B, we showed that AraC promoter is effectively controlled by arabinose, comproving that our system works.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 294
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 1532
Illegal NgoMIV site found at 1589
Illegal NgoMIV site found at 1714
Illegal AgeI site found at 125 - 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI site found at 107
| None |