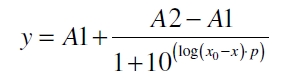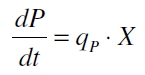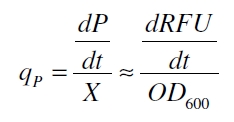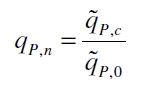Part:BBa_K389016:Experience
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K389016
Transfer function of BBa_K389016
The data for the transfer function was measured and analyzed as described below. The data was fitted with a dose response function of the form
with the hill coefficient p, the bottom asymptote A1, the top asymptote A2 and the inflection point log(x0). Figure 1 shows the measured normalized specific production rates qP,n plotted against the logarithm of the concentration of the inductor acetosyringone in µM. The fit has an R2 = 0.99.
The important data from the transfer function is summarized in table 1:
Table 1: Data from the transfer function for the part BBa_K389016.
| Parameter | Value |
|---|---|
| Hill coefficient | 1.673 |
| Inflection point | 26.5 µM |
| Top asymptote | 2.62 |
Data analysis for BBa_K389016
The data analysis is made in three steps. First step is the processing of the fluorescence raw data gained by the fluorescence plate reader for every sample:
In the second step the RFUcorrected of every sample is plotted against the cultivation time it was drawn. The data is fitted by an exponential fit of the following style:
The accumulation of mRFP in the cells is always exponential. A typical fitted product accumulation curve is shown below:
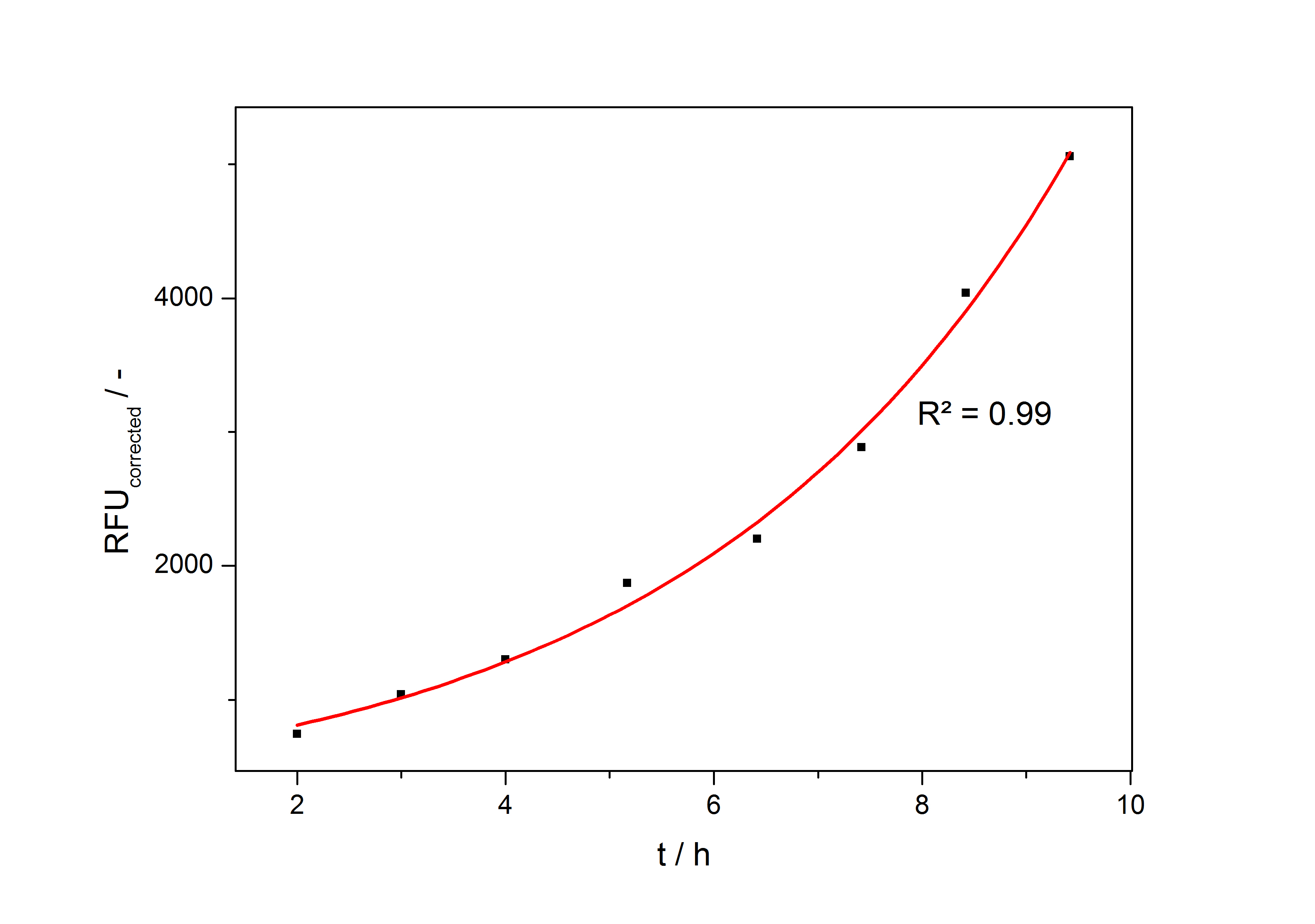
The product accumulation in a cultivation can be described as:
with the amount of product P, the cell count X and the specific production rate qP.
RFU is commensurate to the concentration of mRFP (P) and the OD600 is commensurate to the cell count (X) ([http:/parts.igem.org/Part:BBa_F2620:Experience/Endy/Data_analysis Canton and Labno, 2004]):
With these assumptions it is possible to calculate the specific production rate of mRFP qP in the third step: the specific production rate for every sample of a cultivation is calculated by the derivation of the exponential fit line which describes the accumulation of product in the culture (dRFU/dt) and the measured OD600 data:
The specific production rates qP of all samples of all cultivations made with a specific inductor concentration c are averaged and normalized against the specific production rate of the uninduced system qP,0:
This normalized specific production rate we calculated is commensurate to relative promotor units (RPU) which is commensurate to PoPS (polymerase per seconds) (Canton and Labno, 2004; Pasotti et al., 2009):
Protocols
Cultivation
- Inoculate 10 mL LB containing desired Antibiotic with glycerol stock
- Cultivate over night at 37 °C and 175 rpm
- Measure the OD600
- Prepare shake flasks with LB, antibiotic and different concentrations of the inductor acetosyringone
- For mRFP measurement at least 20 mL starting volume
- Inoculate the main culture with a starting OD600 of 0.1
- Cultivate at 37 °C and 175 rpm
- Take a sample at least every hour and measure the OD600
Measurement
- Take at least 500 µL sample for each measurement (200 µL is needed for one measurement) so you can perform a repeat determination
- Freeze samples at -80 °C for storage
- To measure the samples thaw at room temperature and fill 200 µL of each sample in one well of a black, flat bottom 96 well microtiter plate (perform at least a repeat determination)
- Measure the fluorescence in a platereader (we used a Tecan Infinite® m200 platereader ) with following settings
- 20 sec orbital shaking (1 mm amplitude with a frequenzy of 87.6 rpm)
- Measurement mode: Top
- Excitation: 584 nm
- Emission: 620 nm
- Number of reads: 25
- Manual gain: 150
- Integration time: 20 µs
References
Canton B and Labno A (2004) Data processing of Part BBa_F2620, https://parts.igem.org/Part:BBa_F2620:Experience/Endy/Data_analysis.
Pasotti L, Zucca S, Del Fabbro E (2009) Characterization experiment on BBa_J23100, BBa_J23101, BBa_J23118, https://parts.igem.org/Part:BBa_J23101:Experience.
User Reviews
UNIQf860abf3c172ddbb-partinfo-00000005-QINU UNIQf860abf3c172ddbb-partinfo-00000006-QINU