Promoters/Catalog/Anderson
Description
Members of the Anderson promoter collection are suitable for general protein expression in E. coli and likely other prokaryotes. The collection is known to cover a range of activities so by testing a few promoters it should be possible to find a promoter activity that suits your application. The promoters were recovered from a library screen by Chris Anderson.
Parts J23100 through J23119 are a family of constitutive promoter parts isolated from a small combinatorial library. J23119 is the "consensus" promoter sequence and the strongest member of the family. The NheI and AvrII restriction sites present within these promoter parts make them a scaffold for further modification.
Obtaining Anderson promoter collection
The sequences of the Anderson promoters can be found via the table below. To obtain the physical DNA, we recommend two approaches -
Via de novo synthesis: Since the promoters are short sequences, they can be easily and cheaply ordered as two single-stranded complementary oligo's and annealed. See here for a tutorial on how to construct short parts via oligo annealing.
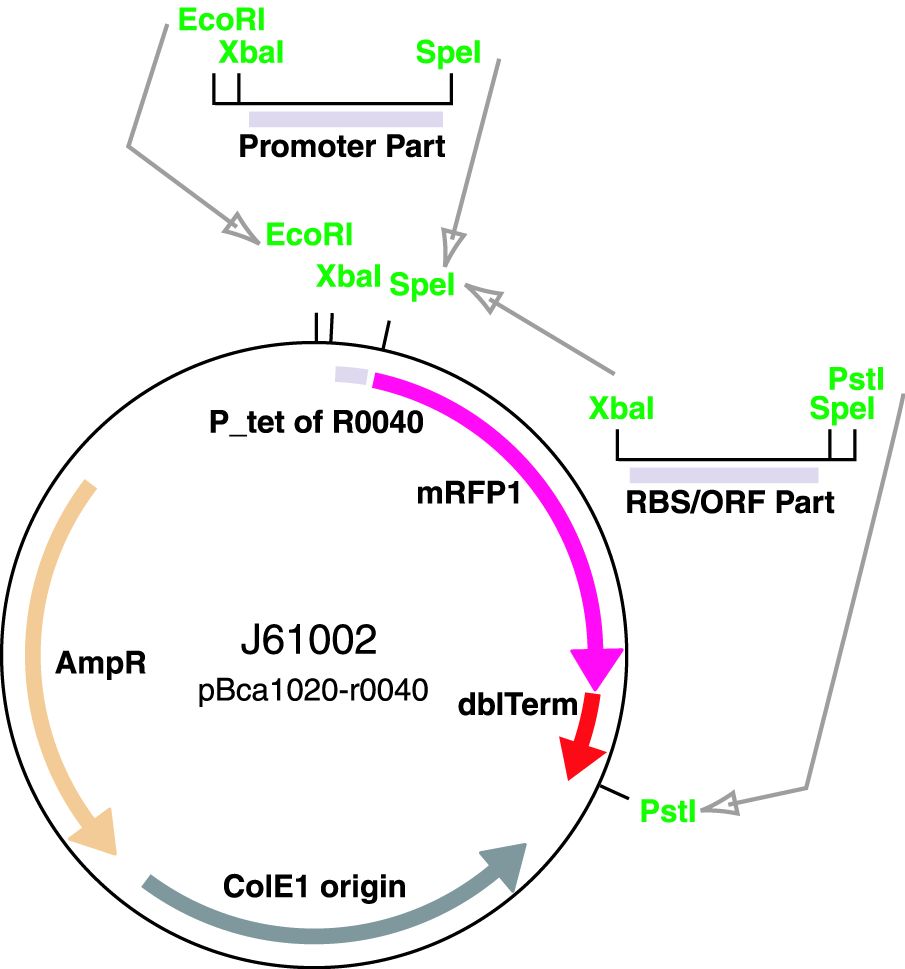
Via the Registry distribution: The promoters are included in the Registry distribution. All parts except BBa_J23119 are present in plasmid BBa_J61002. This places the RFP downstream of the promoter. Part BBa_J23119 is present in pSB1A2.
Anderson promoter collection
J23112 1 J23103 17 J23113 21 J23109 106 J23117 162 J23114 256 J23115 387 J23116 396 J23105 623 J23110 844 J23107 908 J23106 1185 J23108 1303 J23118 1429 J23111 1487 J23101 1791 J23104 1831 J23102 2179 J23100 2547
| Identifier | Sequencea | Predicted Strengthb | |
|---|---|---|---|
| Mean | CV | ||
| BBa_J23100 | TCTAGAGAAAGAGGGGACAAACTAGATG | 1 | |
| BBa_J23101 | TCTAGAGAAAGACAGGACCCACTAGATG | 0.70 | |
| BBa_J23102 | TCTAGAGAAAGATCCGATGTACTAGATG | 0.86 | |
| BBa_J23103 | TCTAGAGAAAGATTAGACAAACTAGATG | 0.01 | |
| BBa_J23104 | TCTAGAGAAAGAAGGGACAGACTAGATG | 0.72 | |
| BBa_J23105 | TCTAGAGAAAGACATGACGTACTAGATG | 0.24 | |
| BBa_J23106 | TCTAGAGAAAGATAGGAGACACTAGATG | 0.47 | |
| BBa_J23107 | TCTAGAGAAAGAAGAGACTCACTAGATG | 0.36 | |
| BBa_J23108 | TCTAGAGAAAGACGAGATATACTAGATG | 0.51 | |
| BBa_J23109 | TCTAGAGAAAGACTGGAGACACTAGATG | 0.04 | |
| BBa_J23110 | TCTAGAGAAAGAGGCGAATTACTAGATG | 0.33 | |
| BBa_J23111 | TCTAGAGAAAGAGGCGATACACTAGATG | 0.58 | |
| BBa_J23112 | TCTAGAGAAAGAGGTGACATACTAGATG | 0.00 | |
| BBa_J23113 | TCTAGAGAAAGAGTGGAAAAACTAGATG | 0.01 | |
| BBa_J23114 | TCTAGAGAAAGATGAGAAGAACTAGATG | 0.10 | |
| BBa_J23115 | TCTAGAGAAAGAAGGGATACACTAGATG | 0.15 | |
| BBa_J23116 | TCTAGAGAAAGACATGAGGCACTAGATG | 0.16 | |
| BBa_J23117 | TCTAGAGAAAGACATGAGTTACTAGATG | 0.06 | |
| BBa_J23118 | TCTAGAGAAAGAGACGAATCACTAGATG | 0.56 | |
aThe sequence of individual promoters are shown in black and red. The grey nucleotides show the bracketing sequence that results from assembling the RBS with an upstream part and a downstream coding sequence. The start codon of the downstream coding sequence is shown in green. bThe relative strengths of these promoters were measured by Chris Anderson and the 2006 Berkeley iGEM team. These measurements are explained in greater detail in the Characterization section below.
Characterization
Measured strengths
Reported activities of the promoters are given as the relative fluorescence of these plasmids in strain TG1 grown in LB media to saturation. See part J61002 for details on their use.