Part:BBa_K1172908
Part 1 of the Biosafety-System Lac of Growth (pRha lacI alr)
Usage and Biology
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 1623
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1547
Illegal BamHI site found at 2249 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 1665
Illegal AgeI site found at 1965 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1422
Functional Parameters
Results
Characterization of the lactose promoter plac
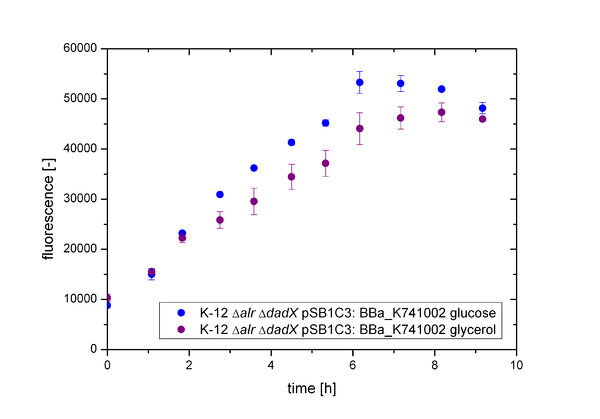
First of all the bacterial growth under the pressure of the unrepressed lactose promoter plac was investigated on different carbon source. Therefore the cultivation on M9 minimal media with Glucose or Glycerol was characterized. To identify the transcription rate of the unrepressed lactose promoter plac the expression of the green fluorescence protein GFP BBa_E0040 behind the plac promoter was used.
As shown in figure 12 below, the bacteria adapted better on glucose then on Glycerol. As glucose is the more powerful energy source, because it posses more carbon atoms than glycerol these result was expected before. So more interesting are the fluorescence measurement shown in the figure 13. As it can be seen also the fluorescence depends on the carbon source, but not as strong as it can be seen by the arabinose promoter [http://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_S pBAD].
This can be explained by the fact that glucose itself also represses the lactose promoter, while glycerol does not and in comparision to the arabinose system the lactose promoter is known to be more leaky. So in the presence of glucose the intracellular concentration of cAMP is low and represses the inefficient catabolism of lactose, so that the glucose is catabolized first by the bacteria resulting in an optimal growth. In the absence of glucose the concentration of cAMP increases, which enhances the transcription of the most operons, who regulate the enzymes for the catabolism of an alternative carbon source. Therefore the expression of GFP under the control of the lactose promoter decreases on glycerol.

So it can be seen that the building of GFP differs between the cultivation on glucose and the cultivation of glycerol. As the production rate by using glucose as carbon source is always beneath the cultivation with glycerol, this impacts that the expression of GFP under the control of the lac promoter is more repressed in the presence of glucose. As the specific production rate was calculated between every single measurement point the curve is not smoothed and so the fluctuations have to be ignored, as they do not stand for are real fluctuations in the transcription in the expression of GFP. They are caused by the growth curve and the fluorescence curve. And as they are not ideal there exists the fluctuations. But this graph shows clearly the difference between the two carbon sources.
Characterization of the Biosafety-System Lac of growth
The Biosafety-System Lac of Growth was characterized on M9 minimal medium with glycerol as carbon source. As for the characterization of the pure lactose promoter above, the bacterial growth and the fluorescence of GFP BBa-E0040 was measured. Therefore the wild type and the Biosafety-Strain E. coli K-12 ∆alr ∆dadX were cultivated once with the induction of 1% L-Rhamnose and once only on glycerol.
First of all it is obviously shown in figure 15 that the growth of the bacteria, who are induced with 1 % L-Rhamnose (blue and black curve) is significant slower than on pure glycerol (orange and red curve). This is attributed to the high metabolic pressure of the induced bacteria. The expression of the repressor lacI and the Alanine-Racemase (alr) simultaneously causes a high outlay of the cells so that they grow slower then the uninduced cells, who expresses only GFP. Additional the lactose promoter is tightly regulated, so that the expression even with a small amount of the repressor lacI is not that high and therefore not as stressful.
Comparing the bacterial growth with the fluorescence in figure 16 it can be seen that the fluorescence of the Biosafety-Strain can not be evaluate because of the long duration of the lac-phase, but the wild-type shows a figure that is comparable with the bacterial growth.
From the figure above it can not be seen if the expression of the repressor lacI does effect the transcription of GFP or not. The slower growth of the bacteria is a first indication that the repressor lacI and the Alanine-Racemase are highly expressed, but as the growth of the bacteria shows nearly the same figure than the fluorescence it could be possible that the repressor does not effect the expression level of GFP under the control of the lactose promoter . That the Biosafety-System works as aspected by repressing the expression of GFP in the presence of L-Rhamnose can be seen from figure 18 below. The calculated specific production rate (equation 1) differs, so that the production of GFP in the presence of L-Rhamnose is always lower than in its absence.
As the specific production rate was calculated between every single measurement point the curve is not smoothed and so the fluctuations have to be ignored, as they do not stand for are real fluctuations in the transcription in the expression of GFP. They are caused by the growth curve and the fluorescence curve. And this measured curves are not ideal the calculation of the specific production rate causes the fluctuations. But it can be seen very clear that the production of GFP differ an is much lower, when the bacteria are induced with 1% L-Rhamnose. So the Biosafety-System Lac of Growth works.
Conclusion of the Results
As the expression level of GFP is increased in the absence of L-Rhamnose and decreased in its presence, the Biosafety-System Lac of Growth works as aspected. In figure 18 the specific production rates after 7,5 hours are compared. It can be seen that the expression level of the lac promoter decreases in the uninduced Safety-Strain compared to the uninduced second part of the Biosafety-System and that the induction with L-Rhamnose leads to a tight repression of the transcription and therefore the expression of GFP.
References
- Baldoma L and Aguilar J (1988) Metabolism of L-Fucose and L-Rhamnose in Escherichia coli: Aerobic-Anaerobic Regulation of L-Lactaldehyde Dissimilation [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC210658/pdf/jbacter00179-0434.pdf|Journal of Bacteriology 170: 416 - 421.].
- Busby S, Ebright RH (1999) Transcription activation by catabolite activator protein (CAP).[http://ac.els-cdn.com/S0022283699931613/1-s2.0-S0022283699931613-main.pdf?_tid=a8f22b74-2cf4-11e3-9457-00000aab0f6c&acdnat=1380891687_f17fd24e5a3e15da96493226fdcaaa10|Journal of molecular biology 239: 199 - 213.].
- Carafa, Yves d'Aubenton Brody, Edward and Claude (1990) Thermest Prediction of Rho-independent Escherichia coli Transcription Terminators - A Statistical Analysis of their RNA Stem-Loop Structures [http://ac.els-cdn.com/S0022283699800059/1-s2.0-S0022283699800059-main.pdf?_tid=ede07e2a-2a92-11e3-b889-00000aab0f6c&acdnat=1380629809_2d1a59e395fc69c8608ab8b5aea842f7|Journal of molecular biology 3: 835 - 858].
- Görke, Boris and Stülke, Jörg (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients [http://www.nature.com/nrmicro/journal/v6/n8/full/nrmicro1932.html|Nature Reviews Microbiology 6: 613 - 624].
- Jacob, F Monod J (1961) Genetic regulatory mechanisms in the synthesis of proteins. [http://www.ncbi.nlm.nih.gov/pubmed/13718526|Journal of molecular biology 216: 318 - 356].
- Lewis, Mitchell (2005) The lac repressor [http://www.sciencedirect.com/science/article/pii/S1631069105000685|Comptes rendues biologies 328: 521 – 548].
- Mossakowska, Danuta E. Nyberg, Kerstin and Fersht, Alan R. (1989) Kinetic Characterization of the Recombinant Ribonuclease from Bacillus amyloliquefaciens (Barnase) and Investigation of Key Residues in Catalysis by Site-Directed Mutagenesis [http://pubs.acs.org/doi/pdf/10.1021/bi00435a033|Biochemistry 28: 3843 - 3850.].
- Paddon, C. J. Vasantha, N. and Hartley, R. W. (1989) Translation and Processing of Bacillus amyloliquefaciens Extracellular Rnase [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC209718/pdf/jbacter00168-0575.pdf|Journal of Bacteriology 171: 1185 - 1187.].
- Voss, Carsten Lindau, Dennis and Flaschel, Erwin (2006) Production of Recombinant RNase Ba and Its Application in Downstream Processing of Plasmid DNA for Pharmaceutical Use [http://onlinelibrary.wiley.com/doi/10.1021/bp050417e/pdf|Biotechnology Progress 22: 737 - 744.].
- Walsh, Christopher (1989) Enzymes in the D-alanine branch of bacterial cell wall peptidoglycan assembly. [http://www.jbc.org/content/264/5/2393.long|Journal of biological chemistry 264: 2393 - 2396.]
- Wickstrum, J.R., Santangelo, T.J., and Egan, S.M. (2005) Cyclic AMP receptor protein and RhaR synergistically activate transcription from the L-rhamnose-responsive rhaSR promoter in Escherichia coli. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1251584/?report=reader|Journal of Bacteriology 187: 6708 – 6719.].
| None |





