Part:BBa_K786031:Experience
Introduction: New Safety Approach
What is BBa_K786031 designed for?
It is designed for a new safety approach for igem and Sythetic Biology. The design based on utilization and adaptation of E.coli’s intrinsic immune system, the CRISPR/Cas system. By re-designing the targeting sequence, we can cleave the transformed plasmid in certain bacteria to reduce the hazard of environment, or even manage to create a controlled suicide system to reduce leakage of such bacteria.
For details, you can refer to CUHK2012's wikipage: [http://2012.igem.org/Team:Hong_Kong-CUHK/SAFETY_APPROACH New Safety Approach]
Expected Result
Firstly, we need to test if the proposed system present in the E. coli. We Used primers to amplify the Cas3 and cascade proteins from E. coli K12 genome DNA. Expected band size of cas3 2.7kbp and cascade 4.4kbp are shown in the gel photo, and sequencing has be done, which proves the existence of these proteins in its genome, thus we can utilize this CRISPR/Cas3 system as a tool for our new safety approach. In the future we can make them into biobrick format and apply into microorganism other than E. coli.
To evaluate whether the RSR system transformed into E. coli can degrade genomic DNA and eventually lead to cell death, we measured OD600 of cell cultures every 0.5 h and plotted the growth curves of transformed E. coli (indicated as solid lines) with or without 0.4 mM IPTG activation. Paired t-test showed that there is no significant difference in OD600 throughput the whole experiment with or without RSR activation by IPTG.
The result agrees with the percentage of survival (indicated as dashed lines) derived from live/dead fluorescent staining assay (Invitrogen) measuring bacterial apoptotic-like rate, which showed no difference in survival percentage with or without RSR activation. It was expected that the survival percentage drops significantly after IPTG induction. Further optimization of design of the RSR region and experimental conditions is required for more specific targeting.
Impact & future applications
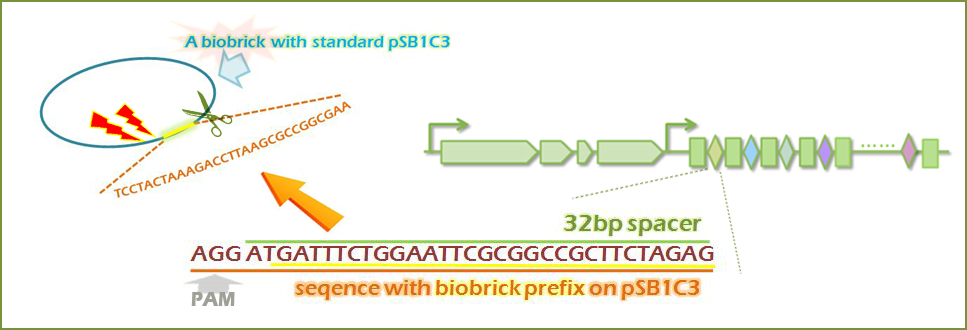
A. Cleavage of selected biobrick
Our proposed system can be utilized as a new safety approach for synthetic biology, especially for iGEMers. As we know, one of the most concerned safety issues in engineering cells is that the leakage of bacteria with transformed plasmid that may cause environmental pollution. As it was proved that CRISPR system can target on plasmid DNA sequence, our system may also function as a tool for biobrick cleavage. Specifically, this tool can be used for targeting and cleaving the antibiotic resistance genes present on the biobricks or the standard site of the biobrick such as prefix, suffix, in order to prevent horizontal gene transfer caused by DNA fragments released from apoptotic pathways. Our system has the potential to be applied as a tool to provide a higher level of the safety control of engineered microorganism machine starting from DNA level.
B, Self-targeting of Engineered Bacteria
Our biobrick has a potential application of cleaving the genome sequence of certain bacteria as selected, which can function as a novel approach to deal with the new safety issue in synthetic biology because by re-designing the segment of sequence for targeting, it can be used to target particular bacterial genome.
With synthetic biology, we can easily apply the system from one organism to another after characterizations. Therefore, by understanding the gene(s) required and the whole mechanism of this biosafety system, it can be engineered into other microorganisms, including the ones lacking native CRISPR system such as Bacillus subtilis. The modification can be done by substituting the promoter, copying the essential machineries from E. coli CRISPR system, and re-designing ‘spacer’ for certain target(s). Theoretically our biobrick can also function in B. subtilis as we choose to express the genes under the Pveg promoter (BBa_K143053), which is a constitutive promoter that works in both E. coli and B. subtilis. Through engineering the E. coli CRISPR/Cas3 system into certain bacteria as well as combining it with different sensors such as light and chemical ones, we may achieve at making a well-regulated system, which can serve as a flexible yet powerful tool for bacteria self-targeting.
User Reviews
UNIQ3d5763d9ffbf2811-partinfo-00000000-QINU UNIQ3d5763d9ffbf2811-partinfo-00000001-QINU