Part:BBa_K731700
Platform for terminators analysis under the control of T7 promoter
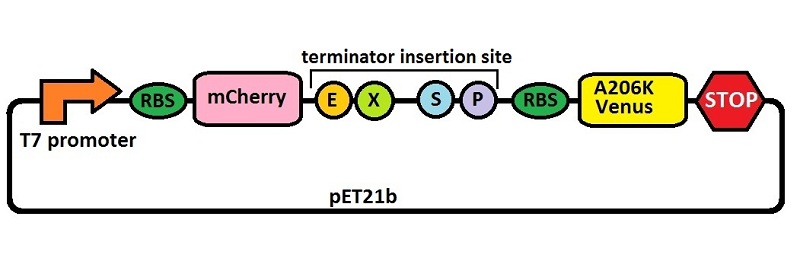
Platform for terminators’ characterization by fluorimetric measurements and ratiometric analysis under IPTG inducible T7 promoter’s control (BBa_J64997). This backbone allows a precise and consistent measure of terminator’s effect on protein expression. It contains a bicistron of fluorescent proteins: mCherry first and then A206K Venus. In between these two genes a BioBrick cloning region has been added, to insert the terminator to be tested. The comparison between the fluorescence of the two proteins with and without the terminator allows to derive some information about the terminator’s effect on protein expression.
An almost identical backbone was also submitted as BBa_K731710. To build it we used a type of insertion-deletion PCR to insert an E. coli tac transcriptional promoter and remove the T7 promoter. The combined use of BBa_K731710 and BBa_K731700 allows also to analyze any potential difference in terminators' activity due to different RNA polymerases.
Usage and Biology
In vivo measurements
Our experiments exploited an E. coli lysogen strain carrying T7 RNA polymerase and lacIq. Additionally, the cells, i.e. E. coli BL21(DE3) pLysS, also contained a plasmid encoding T7 lysozyme and chloramphenicol resistance. T7 lysozyme is a natural inhibitor of T7 RNA polymerase activity, thus reducing background expression of the target genes. The T7 RNA polymerase is behind a lacUV5 promoter.
Experimental set up: Measurements
We made some preliminary analyses to define the best procedure to use this platform. We found that the emission from mVenus was much stronger than the mCherry emission, resulting in some spectral overlap. To improve the quality of the data, off-peak excitation and emission wavelengths were identified that minimized the effect. Therefore, mVenus was excitated at 485 nm (excitation peak is at 515 nm), and the emission of mVenus was collected at its maximum position, i.e. 528 nm. The maximum mCherry excitation wavelength was used (587 nm), but the emission of mCherry was read at 615 nm, as opposed to the maximum at 609 nm. Here is a summary these wavelengths:
| Excitation (nm) | Emission(nm) | |
| mCherry | 587 | 609 |
| Venus | 515 | 528 |
TABLE 1. Excitation and emission wavelengths from literature
| Excitation (nm) | Emission(nm) | |
| mCherry | 587 | 615 |
| Venus | 485 | 528 |
TABLE 2. Excitation and emission wavelengths adapted for the characterizations with this part
The data were collected from cells that were expressed for 4 h with 0.1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). To identify optimal conditions, we screened samples that were and were not sonicated, sample dilutions to decrease the scattering of light, IPTG concentration, time of induction and time after induction. We found that the best results were obtained with induction with 0.5 mM IPTG for 3 h, followed by sonication and centrifugation. The cleared supernatant was then diluted 3-fold with phosphate buffer saline (PBS, composition) and stored overnight at 4 °C. Finally, fluorescence was measured with a Varian Cary Eclipse spectrofluorimeter as described above. The samples were left overnight at 4 °C to allow for sufficient time for the fluorescent proteins to properly mature (i.e. protein folding and chromophore formation). Each measurement was made in quadruplicate from different colonies from different transformations and from different plates.
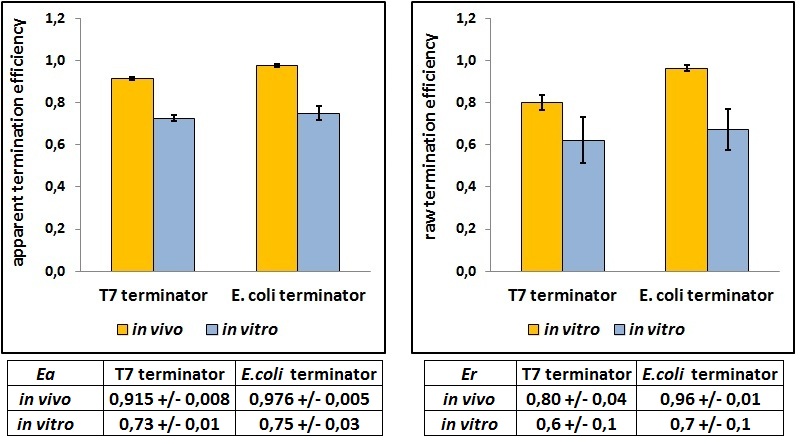
This protocol was used to characterize a T7 wild type terminator (BBa_K731721)and an E. coli terminator (BBa_K731722). These terminators's activity was measured as the ratio between the two proteins'levels using the equation found in literature for termination efficiency. Doing this measurements, however, we realized that terminators can have an effect also on mCherry expression, enhancing it. As a consequence of this unexpected outcome, we defined other two parameters that are here summarized in addition to the literature definition of termination efficiency:
The parameters used to analyze the data are:
apparent termination efficiency as defined in literature, 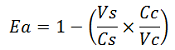
raw termination efficiency, that not consider the mCherry contribution, 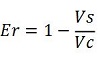
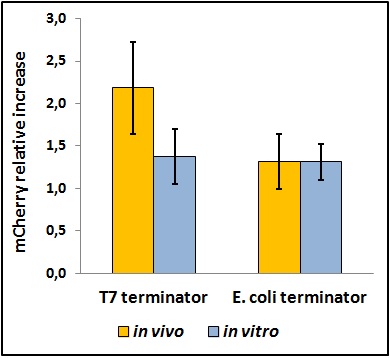
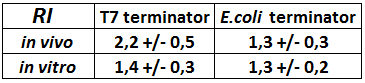
quantification of the rise in mCherry expression caused by the terminator, ![]()
where
-Vs is the A206K Venus peak’s intensity of the construct with the terminator of interest inserted in the prefix-suffix linker
-Vc is the A206K Venus peak’s intensity of the control construct with no terminator
-Cs is the mCherry peak’s intensity of the construct with the terminator inserted
-Cc is the mCherry peak’s intensity of the control construct
In vitro measurements
One potential reason for the increased mCherry levels could result from protection against cellular RNases. Although RNases would not explain why the effect was only observed for the T7 terminator, we decided to test some of the constructs in a purified system that lacked the presence of nucleases. Therefore, we exploited the PURExpress, which consists of purified transcription - translation machinery including T7 RNA polymerase and E. coli ribosomes.
Results
FIGURE 1. BBa_K731721 T7 terminator's and BBa_K731722 E. coli terminator's transcriptional termination efficiencies
The first chart shows the termination efficiencies as usually calculated. The second graph represents the termination efficiencies calculated excluding the mCherry contribution. More specifically, the A206K Venus raw peaks were normalized over the control construct with no intervening terminator.
FIGURE 2. BBa_K731721 T7 terminator's and BBa_K731722 E. coli terminator's effects on mCherry expression
This chart shows the raw emission peaks of mCherry normalized on the control construct without intervening terminator. Hence, the data can be interpreted as the time-folds of mCherry growth caused by the two terminators.
We submitted also this backbone with each terminator used in this study inserted in the cloning region as measurement plasmids; we hope that this could be helpful for anyone who want to verify and further delve into our results. They are BBa_K731701 for the T7 terminator and BBa_K731702 for the E. coli terminator.
More information about the procedure used, the results obtained and our considerations can be found in the Trento iGEM 2012 web page.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Plasmid lacks a prefix.
Plasmid lacks a suffix.
Illegal EcoRI site found at 6850
Illegal SpeI site found at 2
Illegal PstI site found at 16
Illegal NotI site found at 9
Illegal NotI site found at 6856 - 21INCOMPATIBLE WITH RFC[21]Plasmid lacks a prefix.
Plasmid lacks a suffix.
Illegal EcoRI site found at 6850
Illegal BglII site found at 6011
Illegal BamHI site found at 6832
Illegal XhoI site found at 753 - 23INCOMPATIBLE WITH RFC[23]Illegal prefix found at 6850
Illegal suffix found at 2 - 25INCOMPATIBLE WITH RFC[25]Illegal prefix found at 6850
Plasmid lacks a suffix.
Illegal XbaI site found at 6865
Illegal SpeI site found at 2
Illegal PstI site found at 16
Illegal NgoMIV site found at 714
Illegal NgoMIV site found at 1051
Illegal NgoMIV site found at 4231
Illegal NgoMIV site found at 4391
Illegal NgoMIV site found at 5979
Illegal AgeI site found at 6800 - 1000INCOMPATIBLE WITH RFC[1000]Plasmid lacks a prefix.
Plasmid lacks a suffix.
Illegal BsaI site found at 2228
Illegal SapI.rc site found at 3310
//plasmid/measurement
| None |