Part:BBa_K3182108
Contents
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 580
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 598
Introduction
pT7-CBDcipA-sfGFPThis part consists of a cellulose binding domain (CBD) from Clostridium thermocellum cellulose scaffolding protein (CipA) with an sfGFP fused, using a flexible GS-linker (-GGGGSGGGGS-), to the CBDCipA. A thrombin cleavage site (-LVPRGS-) is added to the end of the linker and its breakage will leave a glycine and serine amino acid attached to the N-terminal of the AsPink fusion protein.
An internal BamHI recognition sequence (RS) has been added to enable changeable fusion proteins. BamHI was chosen because its RS codes for glycine and serine, fitting it to the end of the thrombin site. It is also cost-effective enzyme and is unaffected by methylated DNA.
This part can be used to track purification, measure CBD binding ability and report breakage of the thrombinsite.
This part uses an expression system with a T7 promotor (BBa_I719005) as well as a 5'-UTR (BBa_K1758100) region which has been shown to further increase expression in E. coli (BBa_K1758106), ([http://www.ncbi.nlm.nih.gov/pubmed/2676996 Olins et al. 1989]), ([http://www.ncbi.nlm.nih.gov/pubmed/23927491 Takahashi et al. 2013]).
CBDcipA and sfGFP 3D structure
Expression system
The part has a very strong expression with a T7 promotor (BBa_I719005) as well as a 5'-UTR (BBa_K1758100) region which has been shown to further increase expression in E. coli (BBa_K1758106), ([http://www.ncbi.nlm.nih.gov/pubmed/2676996 Olins et al. 1989]), ([http://www.ncbi.nlm.nih.gov/pubmed/23927491 Takahashi et al. 2013]). Both this part and the part were sfGFP was changed for AsPink (BBa_K3182000) showed great expression.
Usage and Biology
The potential usage of this part can be divided in three part. Measurement of CBD binding ability, tracking of purification and reporter of linker breakage.
Measurement of CBD binding ability
Tracking of purification
Reporter of linker breakage
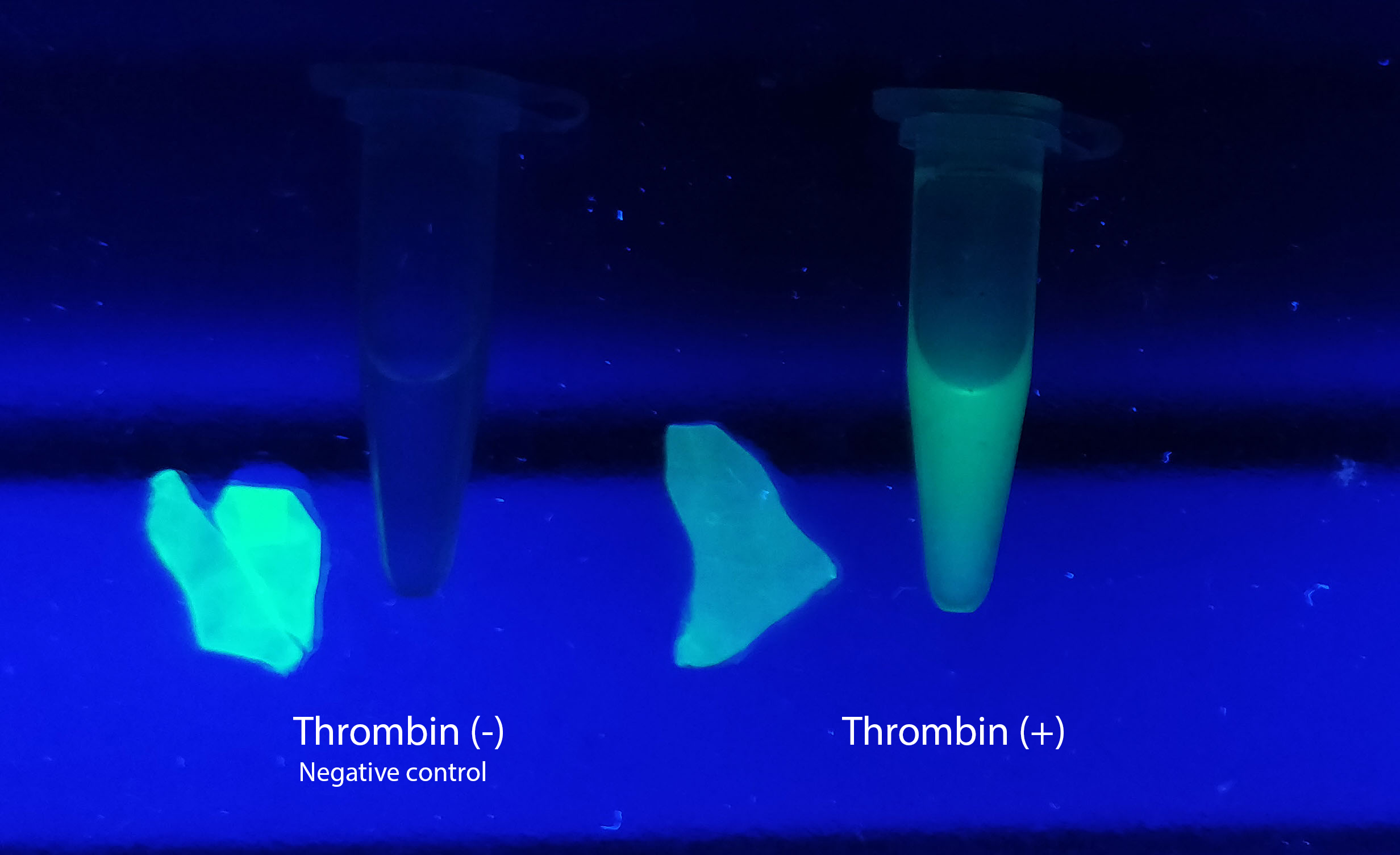
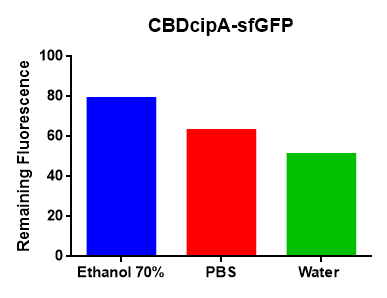

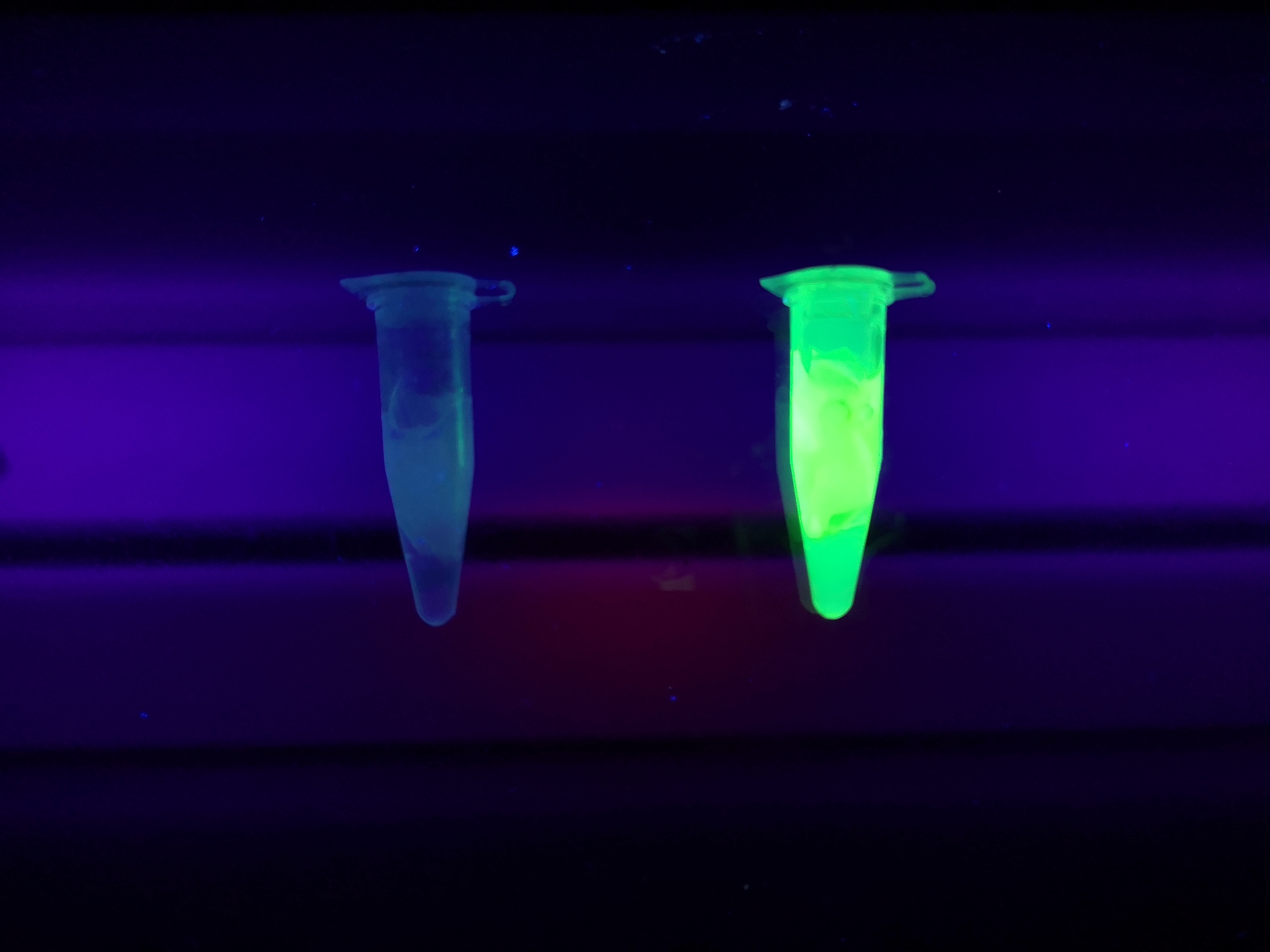
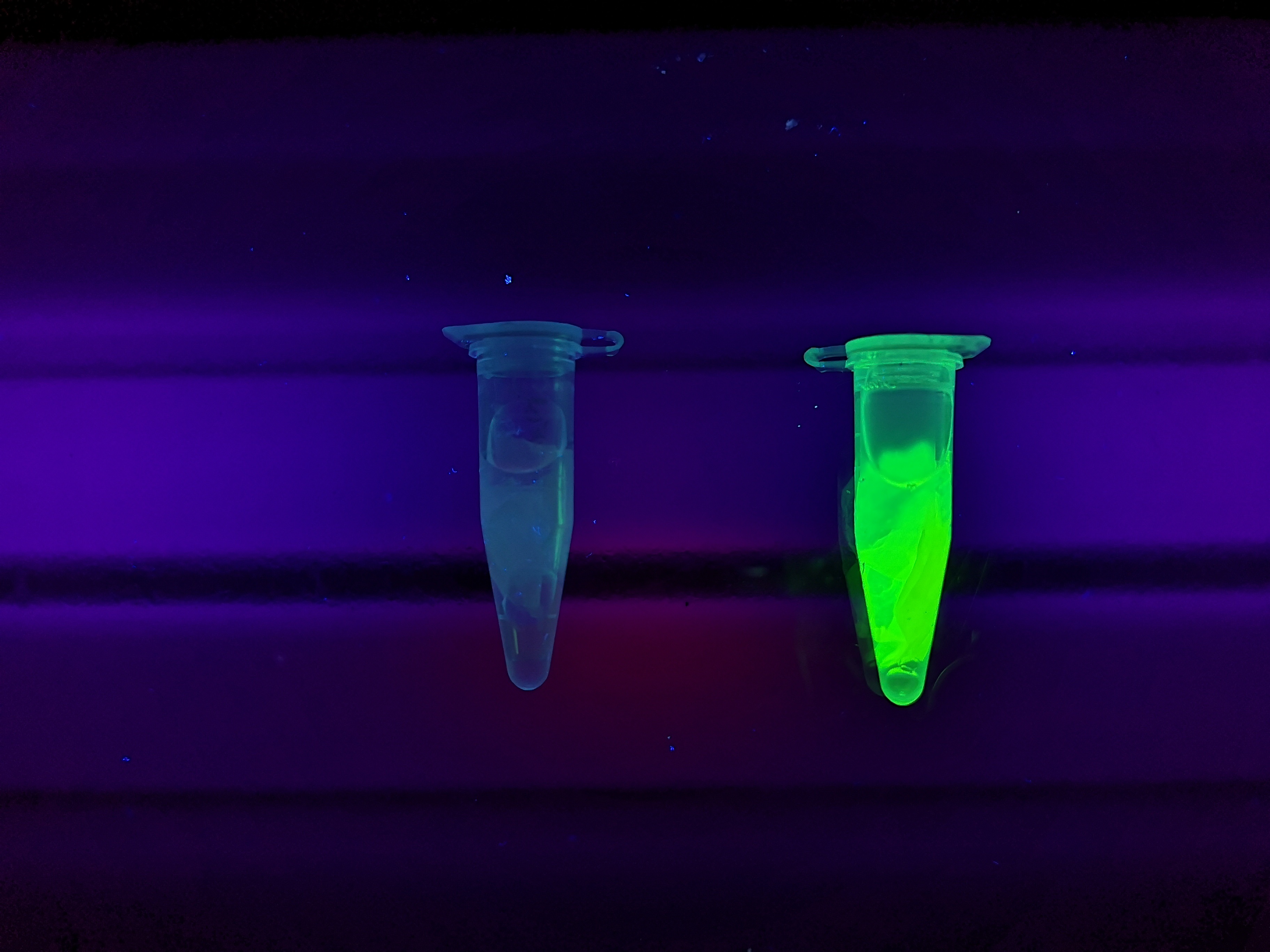
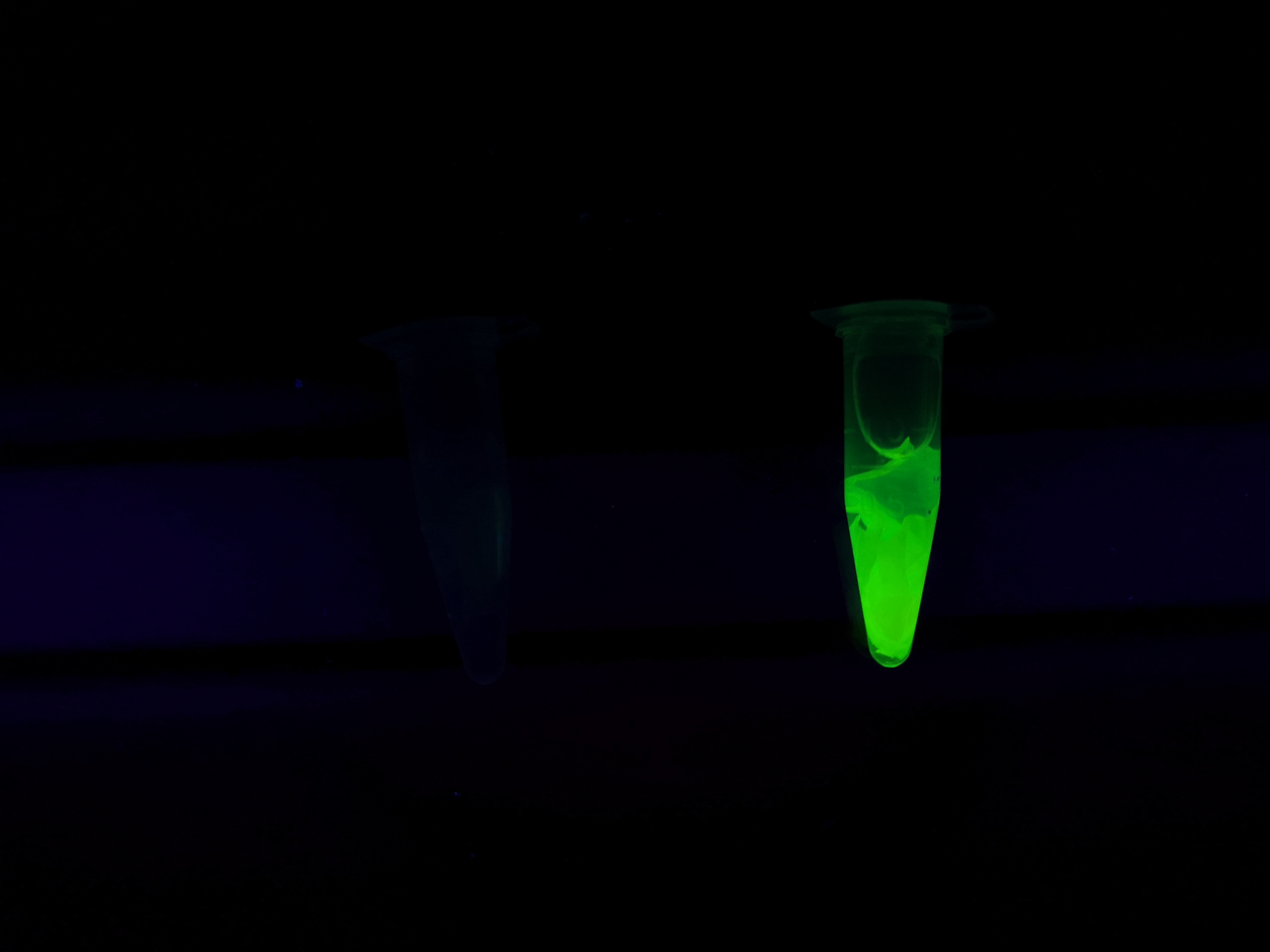
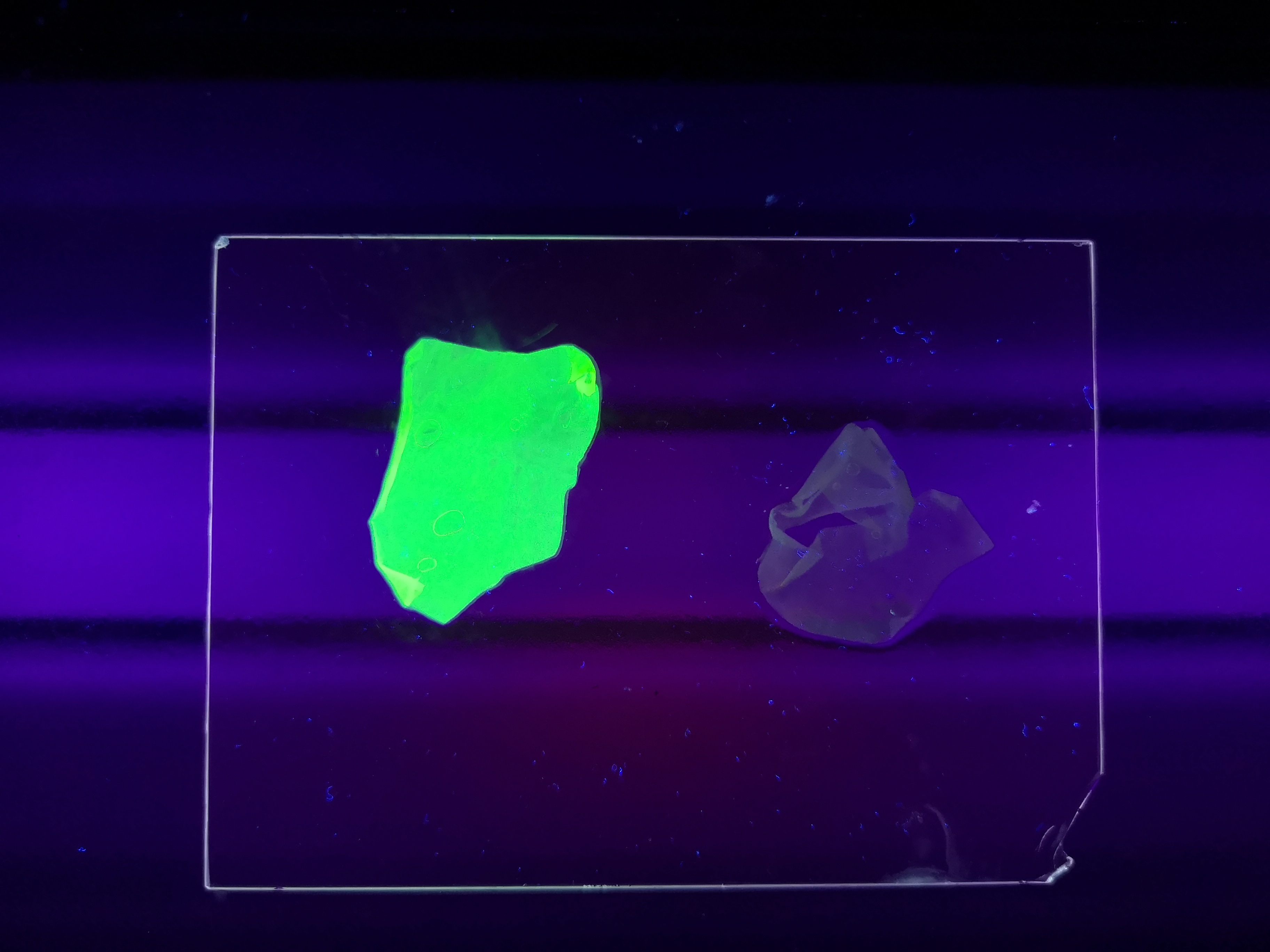 Figure Z Picture 1: Binding studies of the CBDcipA-sfGFP bound to bacterial cellulose. Washed three times with either 70 % ethanol, PBS or deionized water. Picture 2: Induced culture after 16 hours. E. coli BL21 (DE3) cells were grown in prescence of 25 ug/mL chlorampenicol until an OD600 of 0.8 at 37 degrees Celsius, and later induced with 0.5 mM IPTG. The induced culture were then incubated in 16 degrees Celsius for 16 hours. Picture 3: Left: CBDcipA-sfGFP bound to bacterial cellulose in form of a thin film, right: bacterial cellulose reference. Binding of CBDcipA-sfGFP was done the same way as the pictures below.
Figure Z Picture 1: Binding studies of the CBDcipA-sfGFP bound to bacterial cellulose. Washed three times with either 70 % ethanol, PBS or deionized water. Picture 2: Induced culture after 16 hours. E. coli BL21 (DE3) cells were grown in prescence of 25 ug/mL chlorampenicol until an OD600 of 0.8 at 37 degrees Celsius, and later induced with 0.5 mM IPTG. The induced culture were then incubated in 16 degrees Celsius for 16 hours. Picture 3: Left: CBDcipA-sfGFP bound to bacterial cellulose in form of a thin film, right: bacterial cellulose reference. Binding of CBDcipA-sfGFP was done the same way as the pictures below.
Figure A Picture 1: Lysate containing CBDcipA-sfGFP with bacterial cellulose before incubation. Picture 2: Lysate bound to bacterial cellulose after incubation in room temperature for 30 minutes on an end-to-end rotator. Picture 3: Bacterial cellulose after incubation with 70 % ethanol in room temperature for 30 minutes on an end-to-end rotator. All pictures were taken on a UV-table for better visualization of the result.
| None |