Template:HelpPage/Assembly Methods
Contents
Restriction Enzyme Assembly
3A Assembly
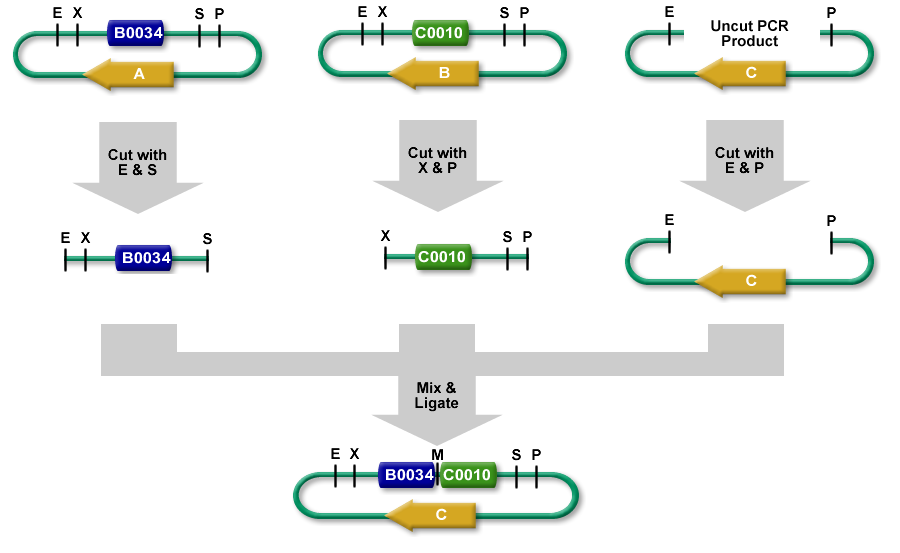
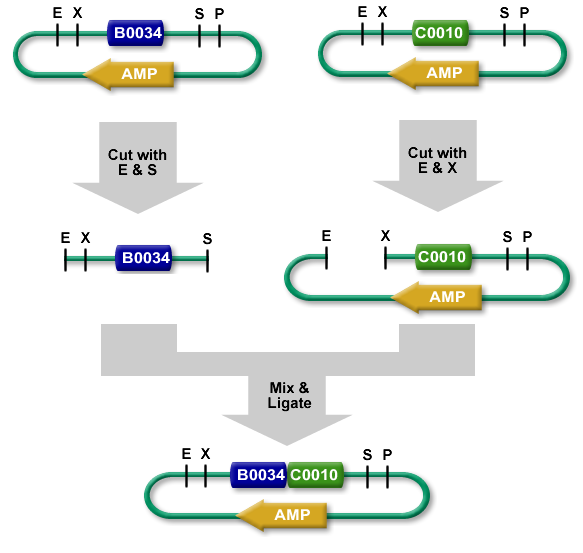
3A Assembly (which stands for three antibiotic assembly) is a method for assembling two part samples and selecting for correct assemblies through antibiotics. 3A assembly uses the restriction sites on the prefix and suffix to assemble part samples. This new composite part will maintain the same prefix and suffix as its "parents" and contain a scar, where the cut and re-ligated restriction sites were stitched together.
It uses effective antibiotic selection to eliminate unwanted background colonies and eliminates the need for gel purification and colony PCR of the resulting colonies. In theory, about 97% of the colonies should be the desired assembly. Additionally, the Registry has been providing iGEM teams and Registry labs with linearized plasmid backbones to further improve this assembly method.
3A Assembly Advantages
- No PCR
- No gel purification
- Higher success rate compared to Standard Assembly.
Links
Type IIS Assembly Methods
Type IIS restriction enzymes, like BsaI, cut DNA outside of their recognition site. This allows for custom overhangs, unlike normal restriction based enzymes (EcoRI cuts at its recognition site creating the same overhang every time). Type IIS assembly methods, such as MoClo, use this to their advantage. Parts will have the same restriction enzyme but distinct custom overhangs, so multiple parts can be assembled at once (one pot assembly).
Type IIs assembly requires careful planning and preparation to ensure the multiple parts will assemble properly and in the correct order. Assembly standards like MoClo, help make this process easier by standardizing part.
Currently, the Registry is evaluating MoClo, to support it as another assembly systems.
Scarless Assembly
There are assembly methods which also allow for the construction of composite parts without scars or specific linkers, which is particularly useful for the assembly of proteins. Additionally, these methods may be able to circumvent assembly standard incompatibility between part samples: a part sample in a Silver RFC[23] plasmid backbone can be assembled with a part sample in a Berkeley RFC[21] plasmid backbone.
DNA Synthesis
Just as DNA synthesis can be used to construct a new basic part, it may also be used to assemble parts together. Using the Registry database you can pull the sequence for parts you're interested, put them together in series, and then send the new composite sequence off to be synthesized.
See the DNA synthesis page for more information and our offer with IDT.
Gibson Assembly
Gibson Assembly has not been tested by the Registry yet, but several teams have had success with this assembly method. The [http://2010.igem.org/Team:Cambridge/Gibson/Introduction Cambridge 2010 iGEM Team] developed a set of protocols and tools that may be useful.
The Registry will be evaluating Gibson Assembly, and will have resources available for this assembly method available soon.
Discontinued Assembly Methods
Standard BioBrick Assembly
For Standard BioBrick Assembly, a part sample is cut out from its plasmid backbone and inserted into the prefix of a plasmid backbone of another part. Two restriction digests are done, one for the part sample that will be moved and one for the plasmid backbone that will receive it. The digests are then run on a gel and using gel purification the required fragments are isolated (the part sample and the cut plasmid backbone). The purified insert and cut plasmid backbone are ligated and the resulting composite part can be transformed into E.coli cells.
Due to its use of gel purification and lower success rate, the Registry no longer recommends the Standard BioBrick Assembly method.