Part:BBa_K2017000
C-split Cas9 + DnaE C-intein
Half of the SpCas9 protein fused to DnaE C-intein. The active full-lenght spCas9 can be recovered by using the other half of the protein, N-split Cas9 + DnaE N-intein (BBa_K2017001). Inteins are used to fuse both parts of the protein, as they interact and splice, leaving the active protein.
Sequence features
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal EcoRI site found at 2020
Illegal PstI site found at 409
Illegal PstI site found at 643
Illegal PstI site found at 1855
Illegal PstI site found at 2159 - 12INCOMPATIBLE WITH RFC[12]Illegal EcoRI site found at 2020
Illegal PstI site found at 409
Illegal PstI site found at 643
Illegal PstI site found at 1855
Illegal PstI site found at 2159 - 21INCOMPATIBLE WITH RFC[21]Illegal EcoRI site found at 2020
Illegal BamHI site found at 203
Illegal XhoI site found at 1741 - 23INCOMPATIBLE WITH RFC[23]Illegal EcoRI site found at 2020
Illegal PstI site found at 409
Illegal PstI site found at 643
Illegal PstI site found at 1855
Illegal PstI site found at 2159 - 25INCOMPATIBLE WITH RFC[25]Illegal EcoRI site found at 2020
Illegal PstI site found at 409
Illegal PstI site found at 643
Illegal PstI site found at 1855
Illegal PstI site found at 2159
Illegal NgoMIV site found at 937 - 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
The protein Cas9 is used in the editing system CRISPR/Cas9. The protein is an endonuclease that cleaves DNA at an specific point, determined by the guide RNA. The guide RNA, which has the structure G-19-NGG, binds the Cas9 and guides it to the DNA strand complementary to the gRNA. Once it recognizes the region to edit, it cuts three nucleotides upstream the recognition sequence, NGG. It is a powerful tool for gene editing.
In this case the Streptococcus pyogenes Cas9 (SpCas9) is the one used in the Valencia UPV iGEM project. The lenght of the Cas9 is 4137bp. We aim to deliver the Cas9 in plants to perform gene editing. As we want to make the editing more efficient, instead of using Agrobacterium tumefaciens as carrier of the Cas9, we use plant viral vectors. However, conventional viral vectors usually are able to carry around 2kb. The SpCas9 is too large. Our solution is to divide the Cas9 in to parts, obtaining a split-Cas9 which can be inserted in viral vector.
To join both parts of the split-Cas9 and reconstitute the functional Cas9 protein, we have used N and C inteins, (BBa_K1362400 and BBa_K1362401, of the 2014 Heidelberg team. These inteins show splicing activity once they join each other. We have added N-intein to the N split-Cas9 (BBa_K2017001) and C-intein to the C split-Cas9, so when they are translated inside the plant cell, the inteins join, splice themselves and let the Cas9 protein complete and fully functional.
Characterization
We introduced the two halves of split-Cas9 inside two different viral vectors (TMV and PVX). These vectors were transformed into Agrobacterium tumefaciens, to avoid the manipulation of viral particles. These vectors were agroinfiltrated into three different Nicotiana benthamianaplants, and it was introduced the corresponding gRNA at different times. In one plant no gRNA was introduced, which was treated as negative control. In the second one gRNA was introduced 3 days after Split-Cas9 system introduction or post infiltration (d.p.i.). In the third plant gRNA was introduced 6 d.p.i. Additionally, we introduced Cas9 endonuclease and gRNA through Ti plasmid into another plant, in order to use it as gene edition positive control.
Time gaps between split-Cas9 and gRNA introduction are due to difference of expression rates among time. Viral system gene expression peaks takes place later than genes introduced through A.tumefaciens Ti plasmid.
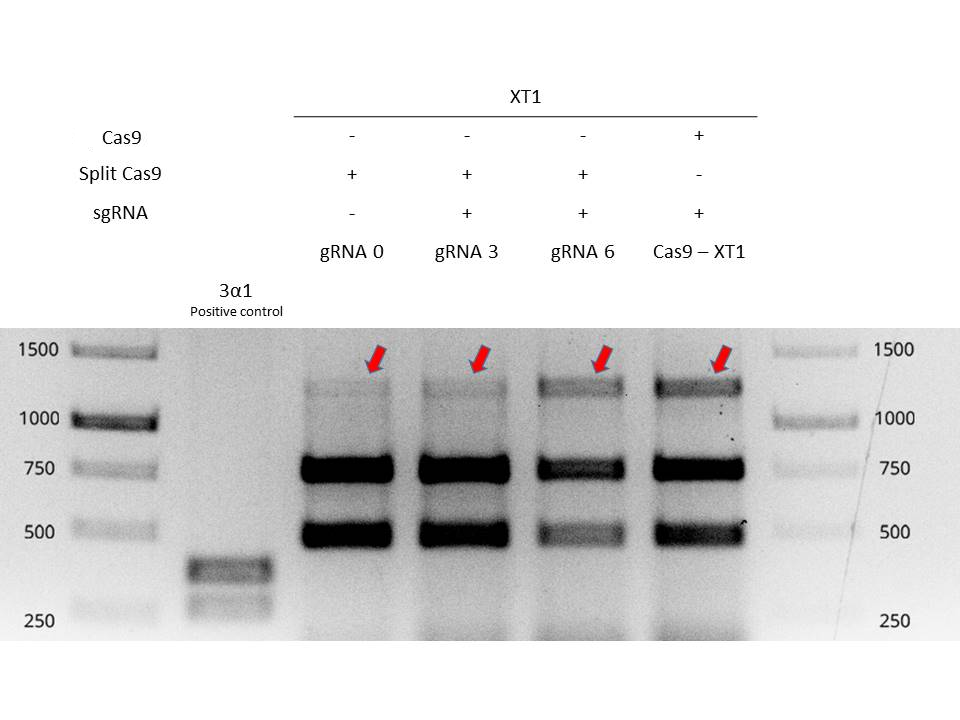
Next, we extract genomic DNA from these four plants and carried out a PCR in order to amplify the mutated region. Afterwards, PCR reactions are purified and digested with EcoRI enzyme in order to determine if mutation has occurred. The target of the gRNA (XT1) is a recognition sequence of the restriction enzyme EcoRI. If the Cas9 has not cut, the EcoRI site is conserved, and the digestion will result in two small bands. If the Cas9 has cut, the EcoRI site will be lost and the enzyme will not cut in the digestion.
In Figure 1 it is shown the digestion of EcoRI of the four plants plus a positive control for the digestion (first column). The 2nd, 3rd, 4th and 5th column show the digestion of the plants agroinfiltrated. The 1st band is the gene XT1 not digested (so the EcoRI site has been lost). The 2nd and 3rd band are the gene XT1 digested. The three bands represent the population of cells that have been edited (1st band) and that have not been edited (2nd and 3rd band).
The Cas9-XT1 column shows our positive control, the complete Cas9 in Agrobacterium with the XT1 gRNA. The 1st band is present. The gRNA0 column shows the split-Cas9 with the gRNA agroinfiltrated the same day as the agroinfiltration of the split-Cas9. The 1st band is much low intense than the positive control, meaning that few cells have been edited. The gRNA3 column shows the split-Cas9 in viral vectors with the gRNA agroinfiltrated 3 days post-infiltration. The 1st band is slighty more intense than gRNA0, so the editing efficiency is higher. Finally, the gRNA6 shows the split-Cas9 in viral vectors with the gRNA agroinfiltrated 6 days post-infiltration. The 1st band is more intense, almost similar to the positive control Cas9-XT1. This means that the split-Cas9 is editing cells, more efficiently if the gRNA is introduced 6 days post-infiltration of the split-Cas9. This is because the viral vectors have had enough time to reproduce and replicate, leaving a high concentration of protein in the cells when the gRNA is infiltrated.
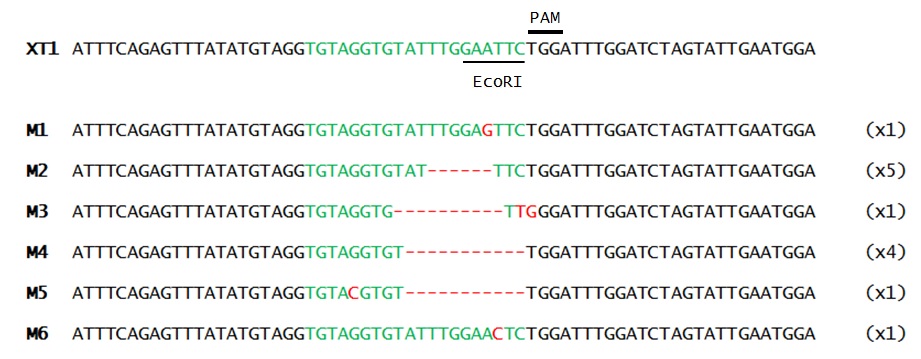
Once the presence of mutations was confirmed, the band resistant to digestion (1st band) was purified, and cloned into a vector. Finally, mutation sequences were analyzed (Fig. 2)
As it can be seen in Figure 2, split-intein-Cas9 system mutation activity delivered through viral vectors was confirmed. Furthermore, as shown in Figure 1, the mutation efficiency of Split-intein-Cas9 seems to be higher when the gRNA is introduced 6 d.p.i. Although its mutation efficiency seems lower than classical Cas9 delivery through Ti plasmid efficiency.
References
Truong D, Kühner K, Kühn R, Werfel S, Engelhardt S, Wurst W et al. Development of an intein-mediated split–Cas9 system for gene therapy. Nucleic Acids Res. 2015;43(13):6450-6458.
Vazquez-Vilar M, Bernabé-Orts J, Fernandez-del-Carmen A, Ziarsolo P, Blanca J, Granell A et al. A modular toolbox for gRNA–Cas9 genome engineering in plants based on the GoldenBraid standard. Plant Methods. 2016;12(1).
Khatodia S, Bhatotia K, Passricha N, Khurana S, Tuteja N. The CRISPR/Cas Genome-Editing Tool: Application in Improvement of Crops. Frontiers in Plant Science. 2016;7.
Li J, Norville J, Aach J, McCormack M, Zhang D, Bush J et al. Multiplex and homologous recombination–mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nature Biotechnology. 2013;31(8):688-691.
Nekrasov V, Staskawicz B, Weigel D, Jones J, Kamoun S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nature Biotechnology. 2013;31(8):691-693.
Shan Q, Wang Y, Li J, Zhang Y, Chen K, Liang Z et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nature Biotechnology. 2013;31(8):686-688.
Functional Parameters
//function/crispr
| None |