Difference between revisions of "Part:BBa K1041000"
(→Sequencing) |
(→Characterisation) |
||
| Line 18: | Line 18: | ||
*<partinfo>BBa_B0015</partinfo>: Double Terminator <br> | *<partinfo>BBa_B0015</partinfo>: Double Terminator <br> | ||
| − | ==Characterisation== | + | ===Characterisation=== |
| − | + | This involved comparisons with the original Bba_J04450 biobrick by performing transformations, restriction digests BLAST analysis and Fluorescent Spectroscopy. We also had our biobrick sequenced. | |
| + | |||
===Transformation=== | ===Transformation=== | ||
| Line 31: | Line 32: | ||
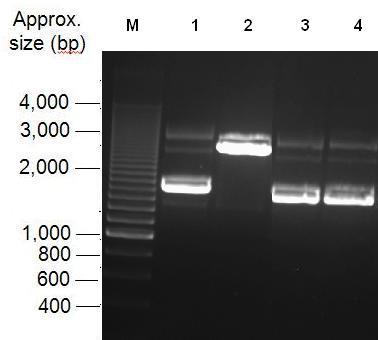
[[image:K1041000 digest 2.JPG|thumb|left|Fig 2:Analysis of Restriction enzyme digest with NdeI. Lanes 1 and 2 contain uncut Bba_K1041000 and Bba_K1041000 digested with NdeI. Lanes 3 and 4 contain uncut Bba_J04450 and Bba_J04450 digested with NdeI respectively.]] | [[image:K1041000 digest 2.JPG|thumb|left|Fig 2:Analysis of Restriction enzyme digest with NdeI. Lanes 1 and 2 contain uncut Bba_K1041000 and Bba_K1041000 digested with NdeI. Lanes 3 and 4 contain uncut Bba_J04450 and Bba_J04450 digested with NdeI respectively.]] | ||
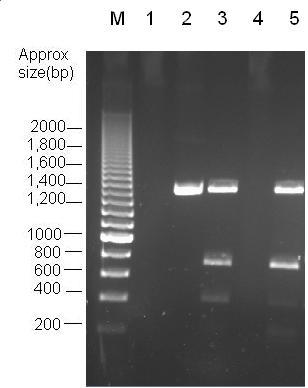
[[image:K1041000 digest.JPG|thumb|centre|Fig 3:Analysis of Restriction enzyme digest with PvuII. Lanes 2 and 3 contain uncut BBa_J04450 and BBa_J04450 digested with PvuII respectively. Lane 5 contains Bba_K1041000 with PvuII.]] | [[image:K1041000 digest.JPG|thumb|centre|Fig 3:Analysis of Restriction enzyme digest with PvuII. Lanes 2 and 3 contain uncut BBa_J04450 and BBa_J04450 digested with PvuII respectively. Lane 5 contains Bba_K1041000 with PvuII.]] | ||
| + | |||
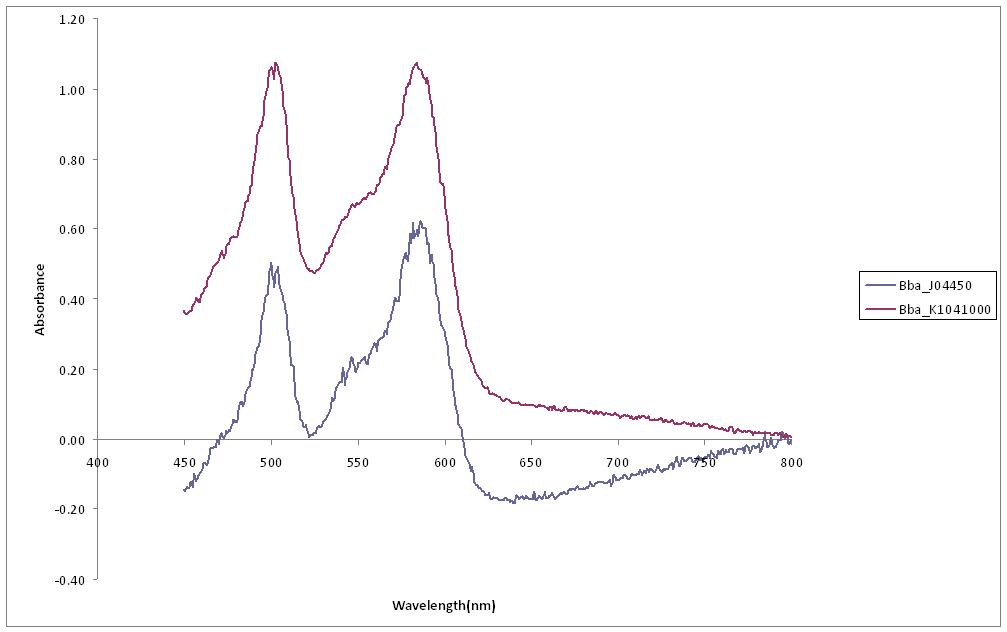
| + | ===Fluorescent Spectroscopy=== | ||
| + | An experiment was designed to compare the intensity of red produced by RFP in part Bba_K1041000 to that produced by Bba_J04450. Both parts were transformed into ''E. Coli'' cells and spread on agar plates containing Chlorophenicol. A culture from each plate was used to inoculate a 10ml LB solution each and left to incubate overnight at 37C. After 24 hours of growth both cultures contained a red colour. The cultures were then analysed using Fluorescent Spectroscopy, ''Fig 4'' to measure the absorbance of the cultures at a range of wavelengths from 450-800nm. | ||
| + | [[image:Fluorescence 1.JPG|500px|Fig 4: Absorbance of cultures containing BBa_J04450 and BBa_K1041000 over a range of wavelengths.]] | ||
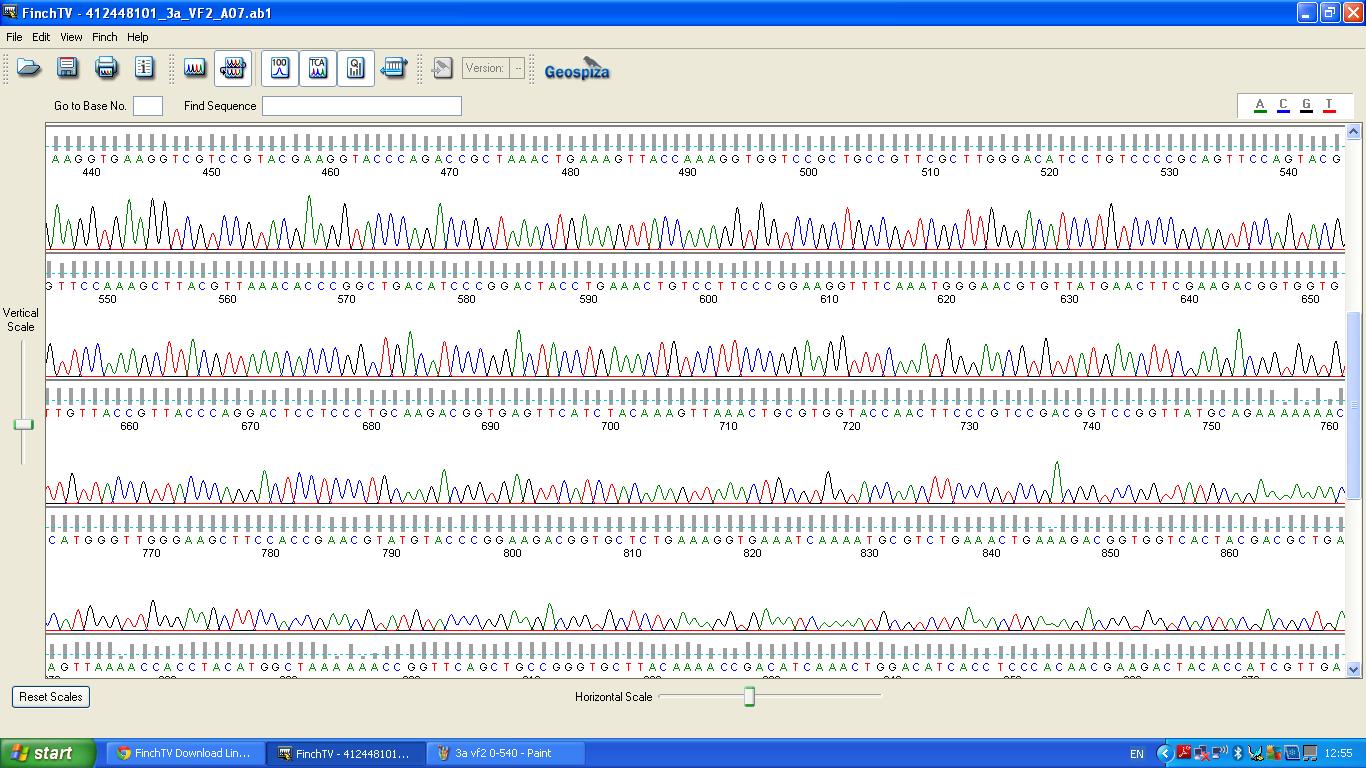
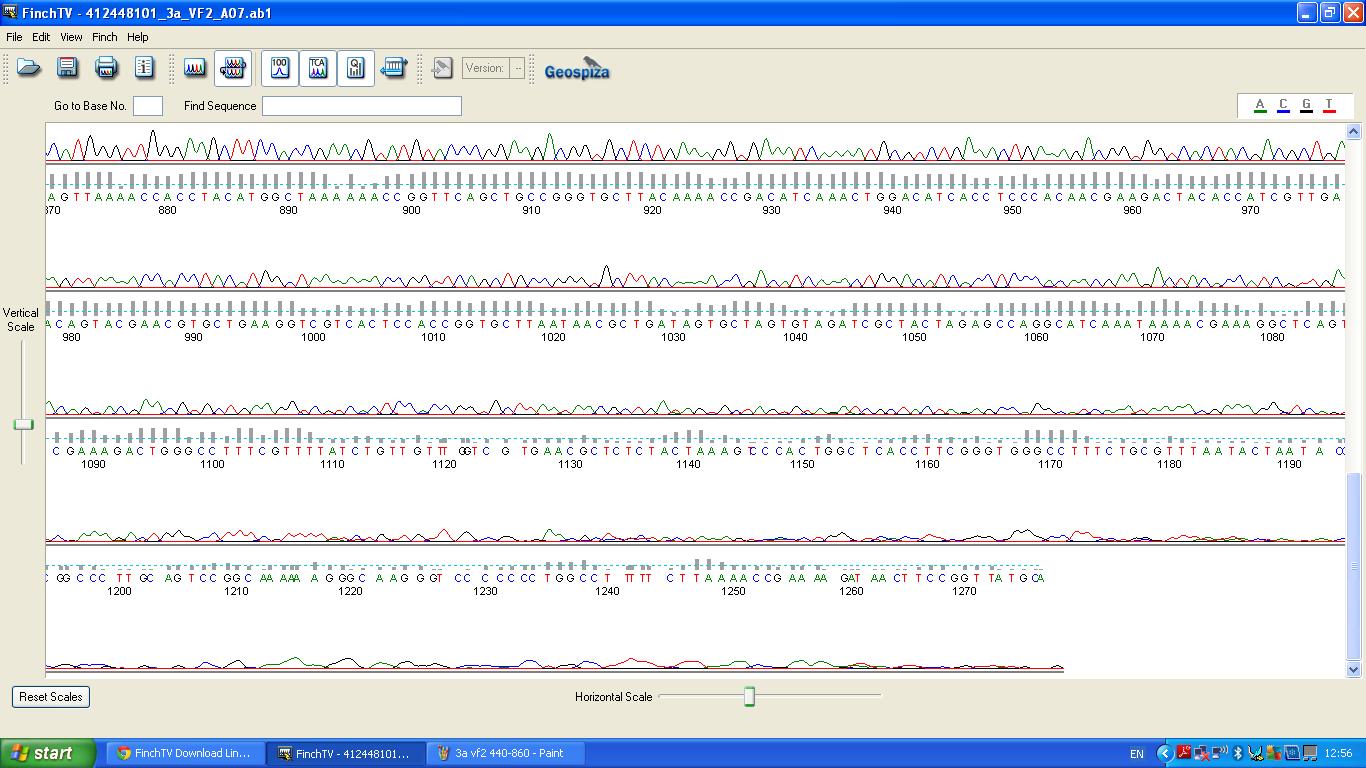
===Sequencing=== | ===Sequencing=== | ||
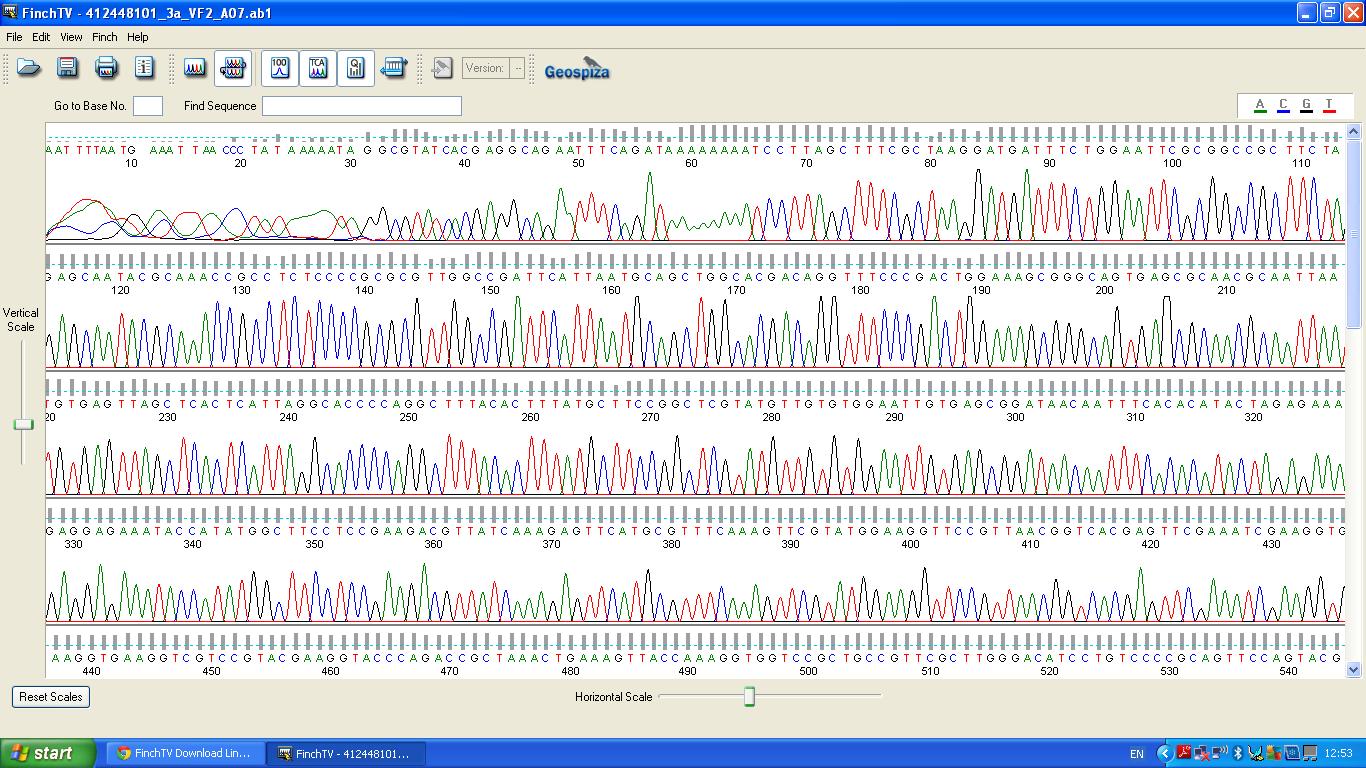
| − | The biobrick was sent off to a company for sequencing, ''Fig | + | The biobrick was sent off to a company for sequencing, ''Fig 4,5,6.''The data we recieved back showed the DNA is of good quality as strong peaks were produced throughout analysis of the sample. |
| − | [[image:3a vf2 0-540.JPG|thumb|left|Fig. | + | [[image:3a vf2 0-540.JPG|thumb|left|Fig. 4:K1041000 sequencing data part 1]] |
| − | [[image:3a vf2 440-860.JPG|thumb|left|Fig. | + | [[image:3a vf2 440-860.JPG|thumb|left|Fig. 5:K1041000 sequencing data part 2 ]] |
| − | [[image:3a vf2 860-.JPG|thumb|left|Fig. | + | [[image:3a vf2 860-.JPG|thumb|left|Fig. 6:K1041000 sequencing data part 3]] |
<br> | <br> | ||
| Line 50: | Line 55: | ||
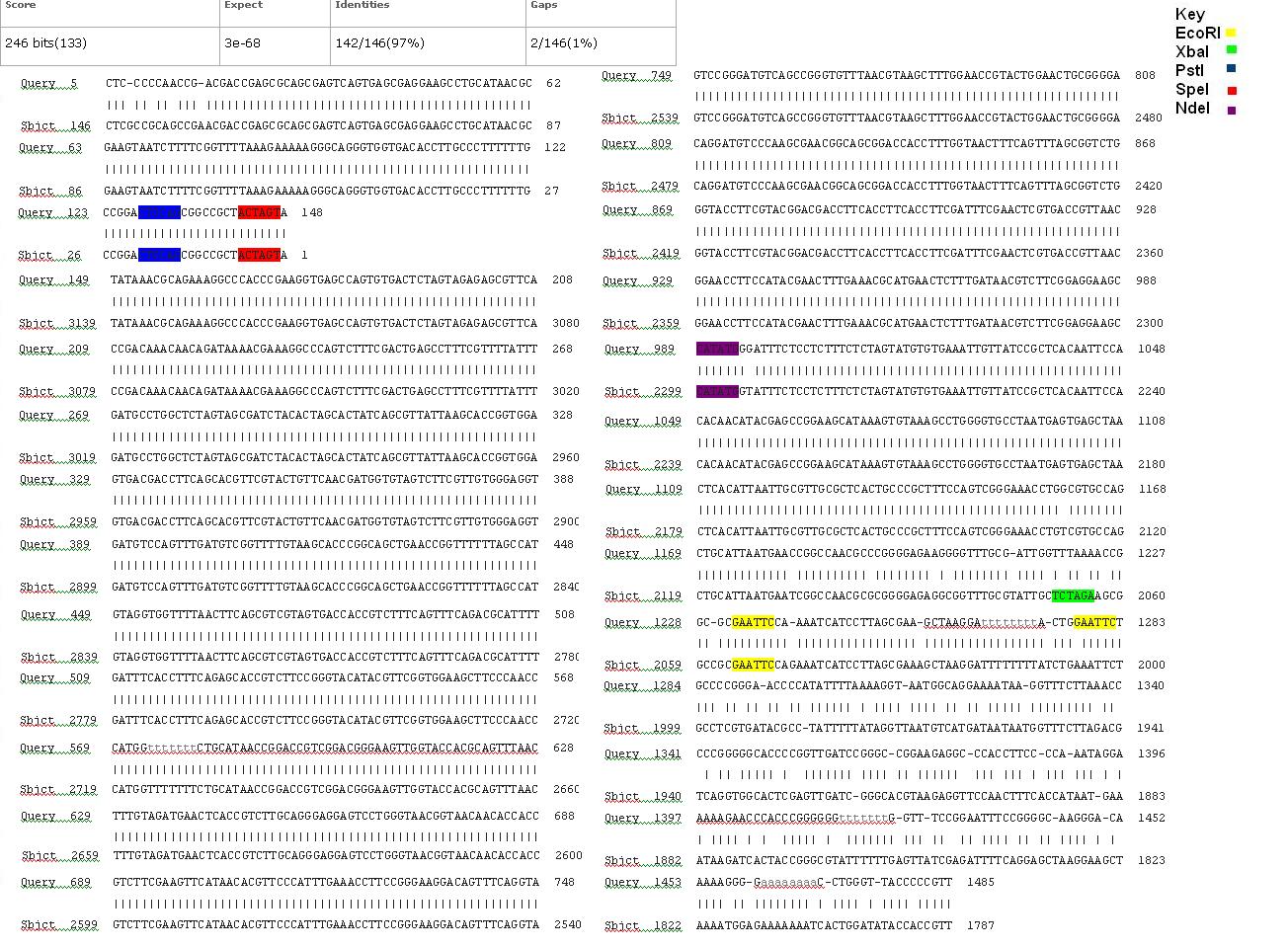
===BLAST Analysis=== | ===BLAST Analysis=== | ||
| − | The data we recieved back from the sequencing company was aligned using BLAST with the expected DNA sequence. The sequencing with both the forward and reverse primers had over 97% matches with the expected DNA sequence. ''Fig | + | The data we recieved back from the sequencing company was aligned using BLAST with the expected DNA sequence. The sequencing with both the forward and reverse primers had over 97% matches with the expected DNA sequence. ''Fig 7,8'' |
| − | [[image:3a Forward.JPG|thumb|left|Fig. | + | [[image:3a Forward.JPG|thumb|left|Fig. 7: Forward primer K1041000 sequencing data aligned with the expected DNA sequence]] |
| − | [[image:3a Reverse.JPG|thumb|left|Fig. | + | [[image:3a Reverse.JPG|thumb|left|Fig. 8: Reverse primer K1041000 sequencing data aligned with expected DNA sequence]] |
Revision as of 15:13, 4 October 2013
RFP Coding Device
Team NRP-UEA_Norwich 2013 added an NdeI restriction site at the start of the RFP coding region of BBa_J04450 using mutagenesis. This was to allow either the promoter region or RFP gene to be excised and exchanged for a different promoter or gene and provide restriction sites for further cloning such as part BBa_K1041002.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 781
Illegal AgeI site found at 893 - 1000COMPATIBLE WITH RFC[1000]
Composite part of the following BioBricks:
- BBa_R0010: Promoter (lacI regulated)
- BBa_B0034: RBS (Elowitz 1999)
- BBa_E1010: Red Fluorescent Protein from Discosoma striata
- BBa_B0015: Double Terminator
Characterisation
This involved comparisons with the original Bba_J04450 biobrick by performing transformations, restriction digests BLAST analysis and Fluorescent Spectroscopy. We also had our biobrick sequenced.
Transformation
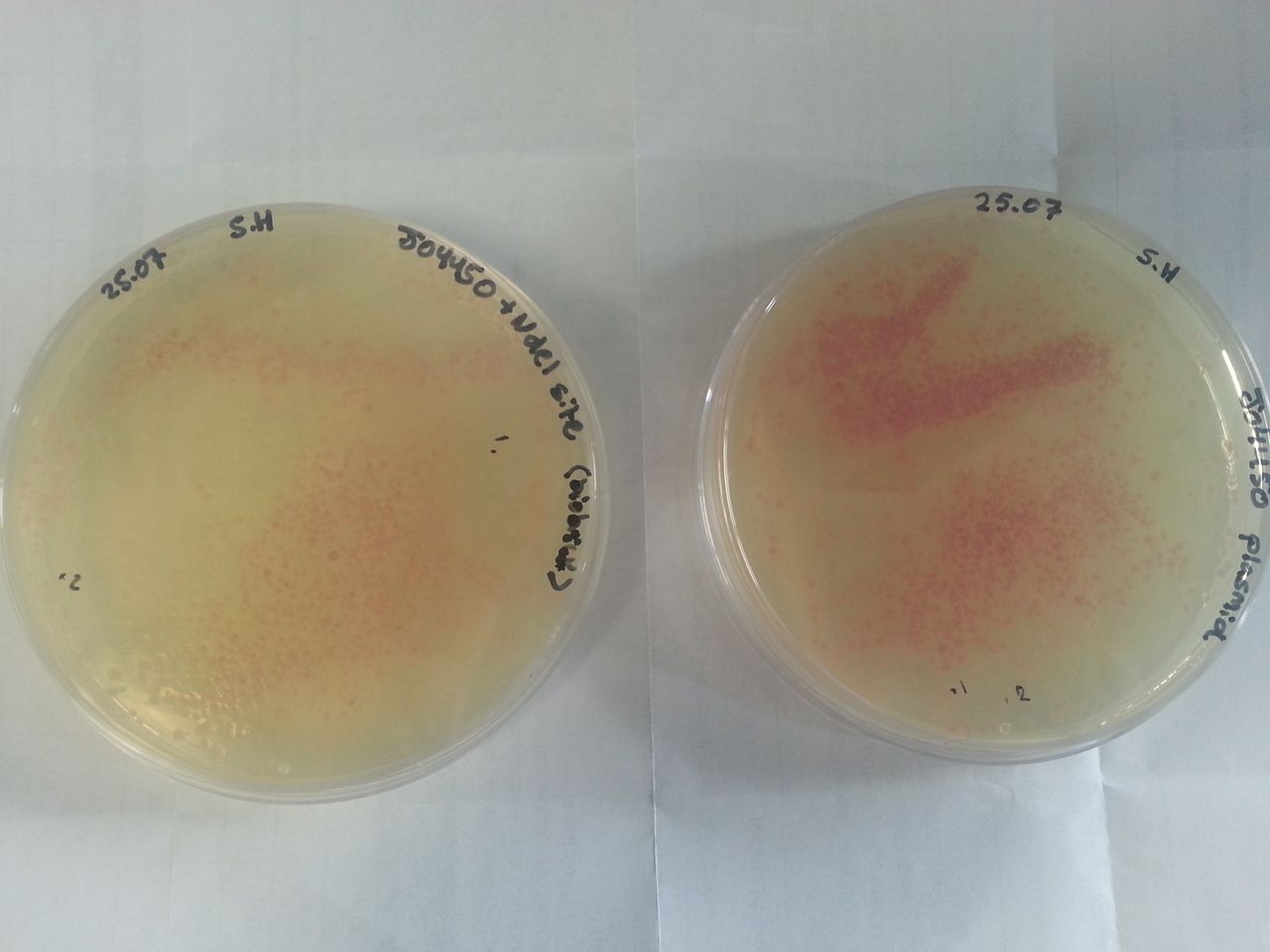
Parts BBa_K1041000 and BBa_J04450 were transformed into Alpha-Select competent E.Coli cells and plated onto LB Agar plates which contained the antibiotic ampicillin. Both plates have colonies that appear red under natural light Fig.1. Therefore the addition of a NdeI site has not effected the ability for the RFP gene to be transcribed.
Restriction Digests
Parts Bba_K1041000 and Bba_J04450 were analysed by restriction digests and run on agarose gels. Both biobricks were cut with NdeI, Fig 2 and PvuII Fig 3. Fig 2 shows that part Bba_K1041000 has an NdeI site and part Bba_J04450 does not as when both parts are cut with NdeI only part Bba_K1041000 produces a different size band in the gel compared to uncut DNA. Fig 3 shows that the rest of the DNA sequence in Bba_K1041000 is identical to Bba_J04450 as bands of the same sized are produced when the parts are cut with the enzyme PvuII.
Fluorescent Spectroscopy
An experiment was designed to compare the intensity of red produced by RFP in part Bba_K1041000 to that produced by Bba_J04450. Both parts were transformed into E. Coli cells and spread on agar plates containing Chlorophenicol. A culture from each plate was used to inoculate a 10ml LB solution each and left to incubate overnight at 37C. After 24 hours of growth both cultures contained a red colour. The cultures were then analysed using Fluorescent Spectroscopy, Fig 4 to measure the absorbance of the cultures at a range of wavelengths from 450-800nm.
Sequencing
The biobrick was sent off to a company for sequencing, Fig 4,5,6.The data we recieved back showed the DNA is of good quality as strong peaks were produced throughout analysis of the sample.
BLAST Analysis
The data we recieved back from the sequencing company was aligned using BLAST with the expected DNA sequence. The sequencing with both the forward and reverse primers had over 97% matches with the expected DNA sequence. Fig 7,8