Difference between revisions of "Part:BBa K525560"
JSchwarzhans (Talk | contribs) (→Bisphenol A degradation with E. coli) |
JSchwarzhans (Talk | contribs) (→Bisphenol A degradation with E. coli) |
||
| Line 22: | Line 22: | ||
===Bisphenol A degradation with ''E. coli''=== | ===Bisphenol A degradation with ''E. coli''=== | ||
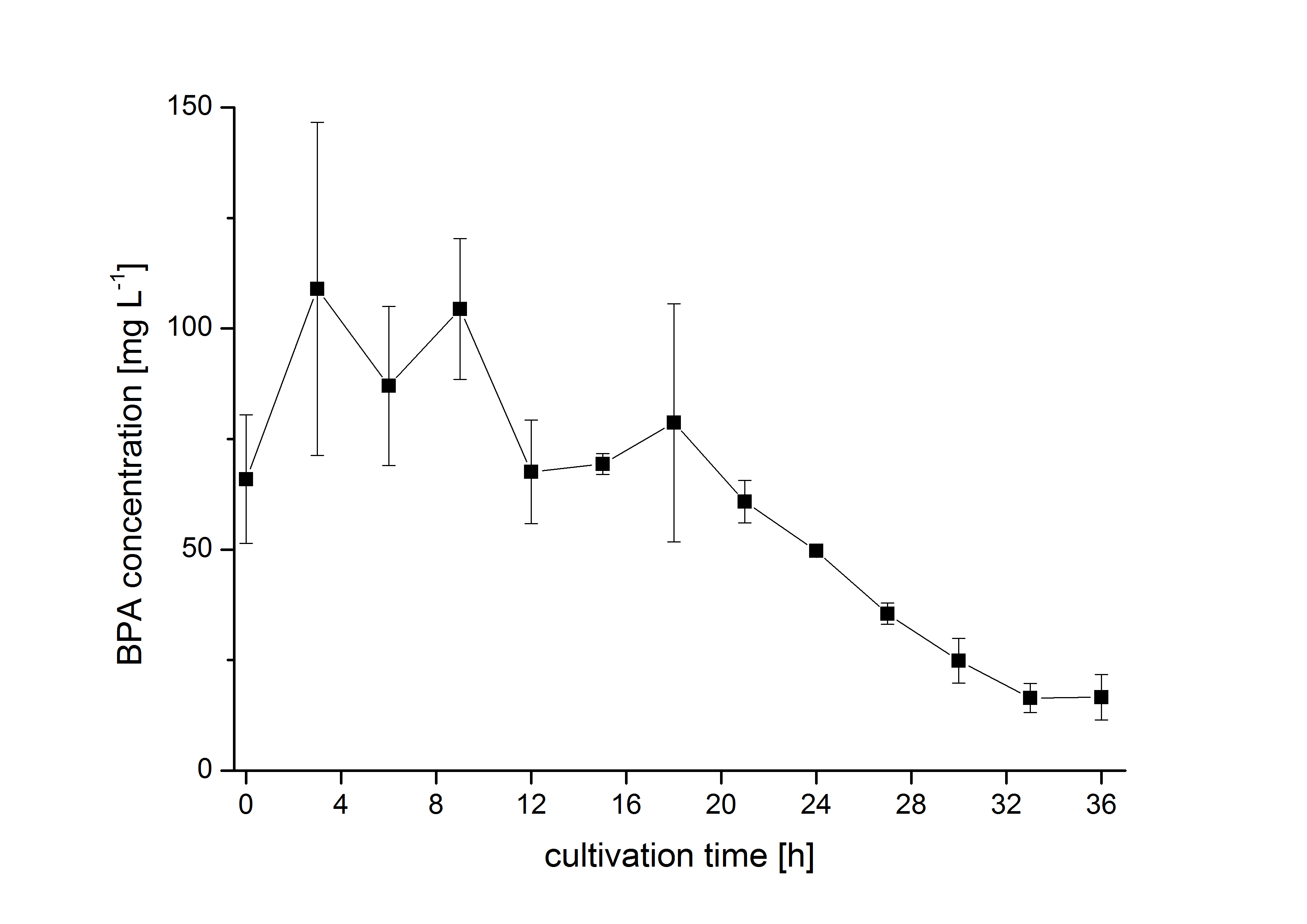
| − | The bisphenol A degradation with the BioBricks <partinfo>K123000</partinfo>, <partinfo>K123001</partinfo> and <partinfo>K525499</partinfo> works in ''E. coli'' KRX in general. Because [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.2008.03843.x/full Sasaki ''et al.'' (2008)] reported problems with protein folding in ''E. coli'' which seem to avoid a complete BPA degradation, we did not cultivate at 37 °C and we did not use the strong T7 promoter as [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.2008.03843.x/full Sasaki ''et al.'' (2008)] did for expressing these BioBricks but we cultivated at 30 °C and we used a medium strong constitutive promoter (<partinfo>J23110</partinfo>). 30 °C is in addition the cultivation temperature of ''S. bisphenolicum'' AO1. With this promoter upstream of the gene expressing the bisdA | bisdB |FNR fusion protein we were able to degrade a substantial amount (~85%) of BPA in about 36 h starting at 120 mg L<sup>-1</sup> BPA . This data is shown in the following figure and indicates that the fusion protein of all three : | + | The bisphenol A degradation with the BioBricks <partinfo>K123000</partinfo>, <partinfo>K123001</partinfo> and <partinfo>K525499</partinfo> works in ''E. coli'' KRX in general. Because [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.2008.03843.x/full Sasaki ''et al.'' (2008)] reported problems with protein folding in ''E. coli'' which seem to avoid a complete BPA degradation, we did not cultivate at 37 °C and we did not use the strong T7 promoter as [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.2008.03843.x/full Sasaki ''et al.'' (2008)] did for expressing these BioBricks but we cultivated at 30 °C and we used a medium strong constitutive promoter (<partinfo>J23110</partinfo>). 30 °C is in addition the cultivation temperature of ''S. bisphenolicum'' AO1. With this promoter upstream of the gene expressing the bisdA | bisdB |FNR fusion protein we were able to degrade a substantial amount (~85%) of BPA in about 36 h starting at 120 mg L<sup>-1</sup> BPA . This data is shown in the following figure and indicates that the fusion protein of all three enzymes that are involved in the degradation of BPA is functional: |
[[Image:Bielefeld_2011_562_BPA_Degradation.png|650px|center|thumb| '''Figure 2: BPA degradation by ''E. coli'' KRX carrying genes for the fusion protein of BisdA, BisdB and FNR behind the medium strong constitutive promoter <partinfo>J23110</partinfo> with RBS <partinfo>B0034</partinfo>. [[Part:BBa_K525517#Methods | Cultivations]] were carried out at 30 °C in LB + Amp + BPA medium for 36 h, respectively, with automatic sampling every three hours in 300 mL shaking flasks without baffles with silicon plugs. Two biological replicates were analyzed.''']] | [[Image:Bielefeld_2011_562_BPA_Degradation.png|650px|center|thumb| '''Figure 2: BPA degradation by ''E. coli'' KRX carrying genes for the fusion protein of BisdA, BisdB and FNR behind the medium strong constitutive promoter <partinfo>J23110</partinfo> with RBS <partinfo>B0034</partinfo>. [[Part:BBa_K525517#Methods | Cultivations]] were carried out at 30 °C in LB + Amp + BPA medium for 36 h, respectively, with automatic sampling every three hours in 300 mL shaking flasks without baffles with silicon plugs. Two biological replicates were analyzed.''']] | ||
Revision as of 13:22, 28 October 2011
Fusion protein of NADP+ Oxidoreductase and BisdA and BisdB
Fusion protein of ferredoxin-NADP+ oxidoreductase, BisdA and BisdB; RFC 25 (Freiburg BioBrick assembly standard) for characterization of intra- and extracellular BPA degradation.
Usage and Biology
Expressing this BioBrick in E. coli enables the bacterium to degrade the endocrine disruptor bisphenol A (BPA).
BPA is mainly hydroxylated into the products 1,2-Bis(4-hydroxyphenyl)-2-propanol and 2,2-Bis(4-hydroxyphenyl)-1-propanol. In S. bisphenolicum AO1, a total of three genes are responsible for this BPA hydroxylation: a cytochrome P450 (CYP, bisdB), a ferredoxin (Fd, bisdA) and a ferredoxin-NAD+ oxidoreductase (FNR) Sasaki05a. The three gene products act together to reduce BPA while oxidizing NADH + H+. The cytochrome P450 (BisdB) reduces the BPA and is oxidized during this reaction. BisdB in its oxidized status is reduced by the ferredoxin (BisdA) so it can reduce BPA again. The oxidized BisdA is reduced by a ferredoxin-NAD+ oxidoreductase consuming NADH + H+ so the BPA degradation can continue Sasaki05a. This electron transport chain between the three enzymes involved in BPA degradation and the BioBricks needed to enable this reaction in vivo and in vitro are shown in the following figure (please have some patience, it's an animated .gif file):
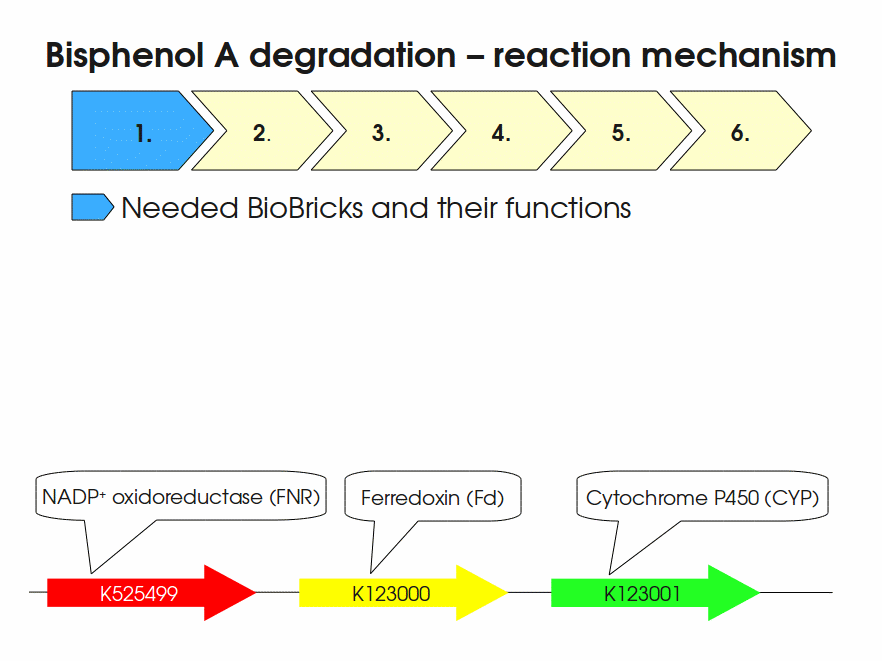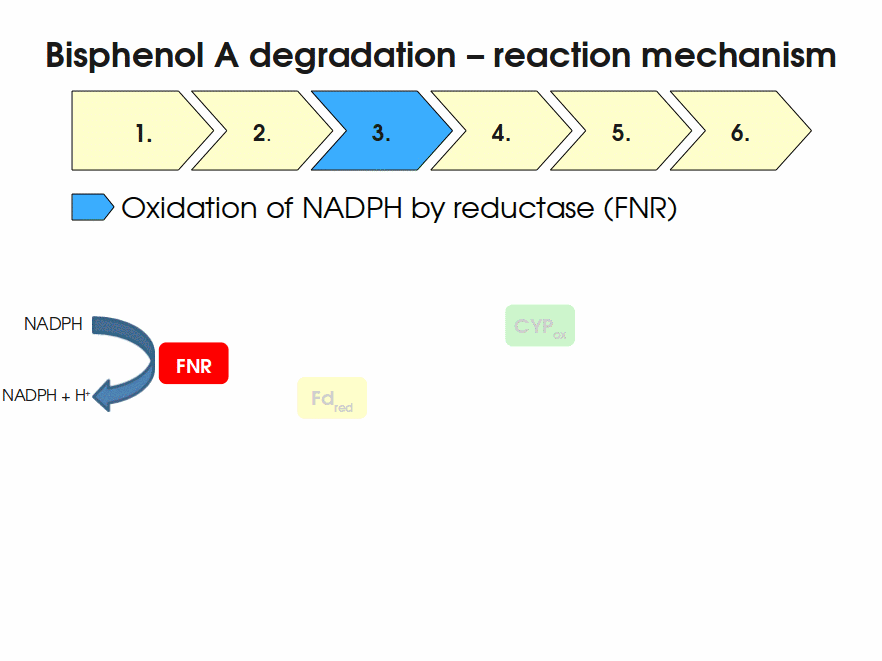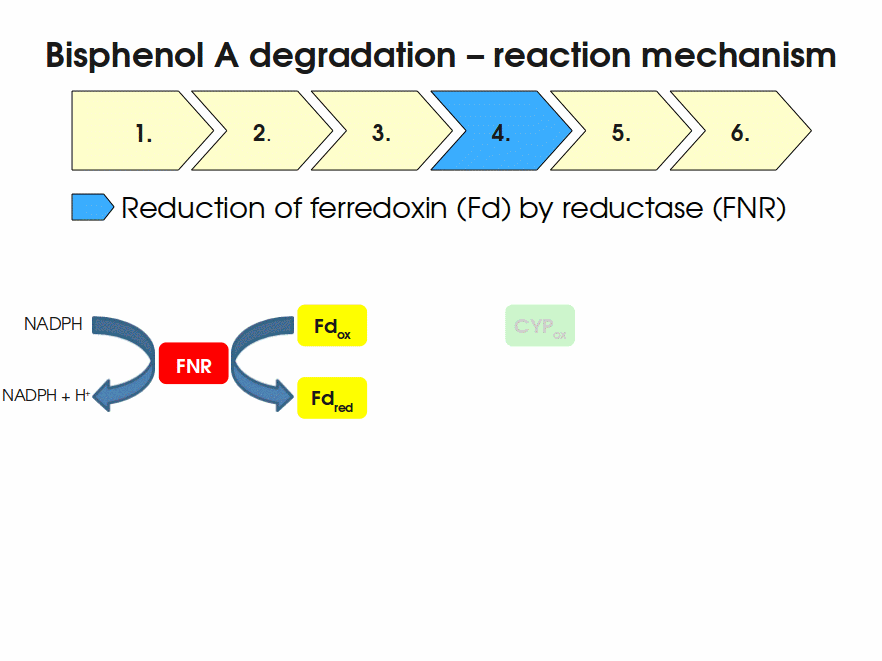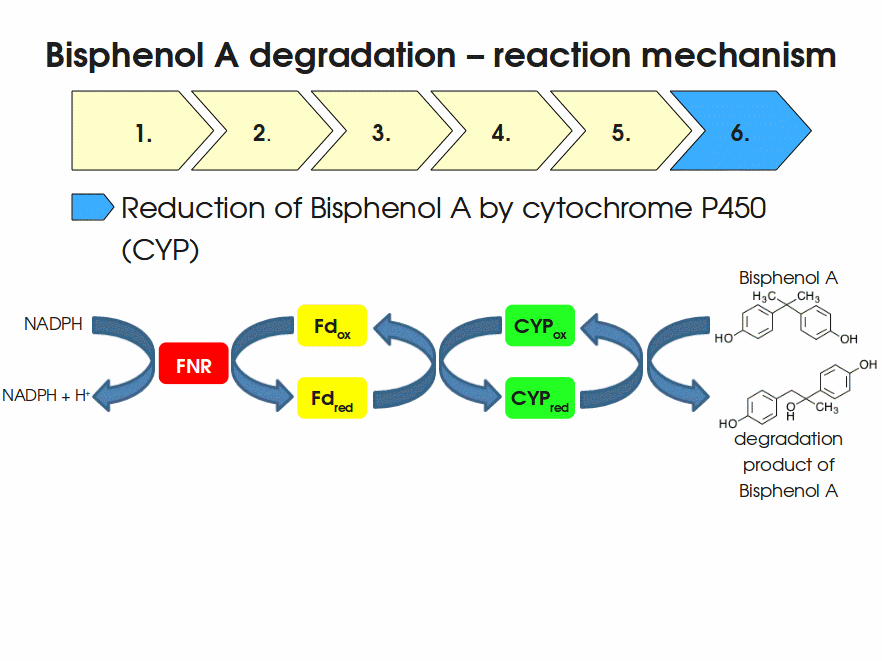
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 1350
Illegal BamHI site found at 2088 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 4
Illegal AgeI site found at 2341 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 685
Bisphenol A degradation with E. coli
The bisphenol A degradation with the BioBricks BBa_K123000, BBa_K123001 and BBa_K525499 works in E. coli KRX in general. Because [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.2008.03843.x/full Sasaki et al. (2008)] reported problems with protein folding in E. coli which seem to avoid a complete BPA degradation, we did not cultivate at 37 °C and we did not use the strong T7 promoter as [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.2008.03843.x/full Sasaki et al. (2008)] did for expressing these BioBricks but we cultivated at 30 °C and we used a medium strong constitutive promoter (BBa_J23110). 30 °C is in addition the cultivation temperature of S. bisphenolicum AO1. With this promoter upstream of the gene expressing the bisdA | bisdB |FNR fusion protein we were able to degrade a substantial amount (~85%) of BPA in about 36 h starting at 120 mg L-1 BPA . This data is shown in the following figure and indicates that the fusion protein of all three enzymes that are involved in the degradation of BPA is functional:
References
<biblio>
- Sasaki pmid=18492046
- Sasaki05a pmid=16332782
</biblio>
