Difference between revisions of "Part:BBa K4115039"
(→Results) |
|||
| Line 41: | Line 41: | ||
===Results=== | ===Results=== | ||
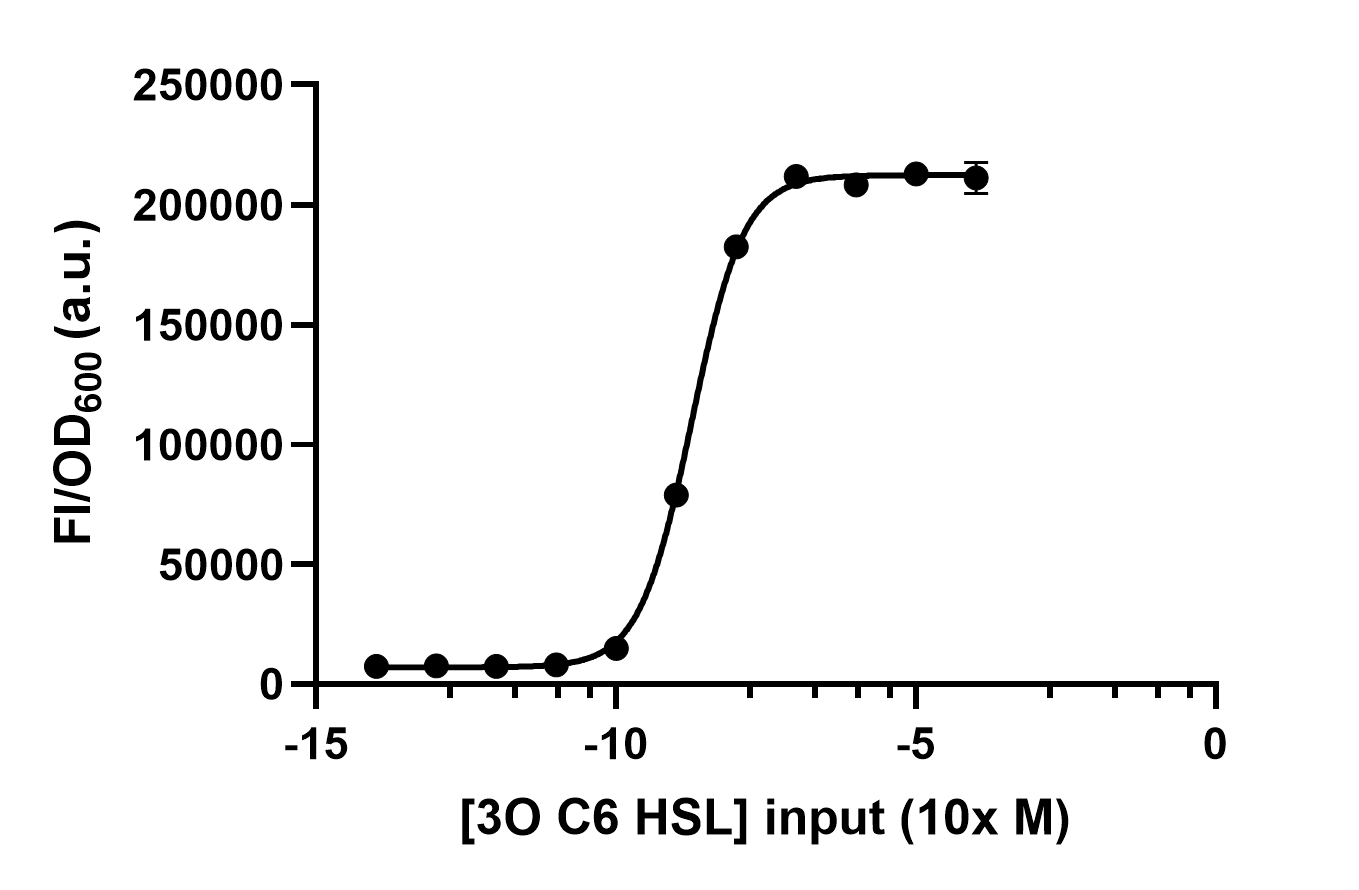
| − | We | + | We process the data by dividing the fluorescence intensity by the OD600 and get Figure 2. Thus, we can conclude the response intensity of LuxR to 3O6C HSL at different concentrations. |
[[File:LuxR receive under different AHL concentrations input.jpg|500px|thumb|left|'''Figure 2:''' Different 3O6C HSL concentrations are inputed to test LuxR response intensity.]]<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | [[File:LuxR receive under different AHL concentrations input.jpg|500px|thumb|left|'''Figure 2:''' Different 3O6C HSL concentrations are inputed to test LuxR response intensity.]]<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
Revision as of 14:29, 4 October 2022
J23101-B0034-luxR-B0015-Lux pR-B0034-sfGFP-B0015
| J23101-B0034-luxR-B0015-Lux pR-B0034-sfGFP-B0015 | |
|---|---|
| Function | Gene regulator |
| Use in | E. coli |
| RFC standard | RFC 10, RFC 10 compatible |
| Backbone | pBBR1, pUC57 |
| Submitted by | ShanghaiTech_China |
This composite part is used to detect the response of LuxR (BBa_C0062) to AHL signaling molecules [1]. When LuxR receives the signal of AHL, it can regulate the downstream Lux pR (BBa_R0062) to make superfolder GFP (BBa_K4115000) express.
This part is togther used with LuxI (BBa_C0161), an AHL(3OC6 HSL here) sender.We can transform the plasmid into the A.caulinodans ORS571 to construct communications and regulations system between E. coli and S.elongatus HL7942 through quorum sensing signals.
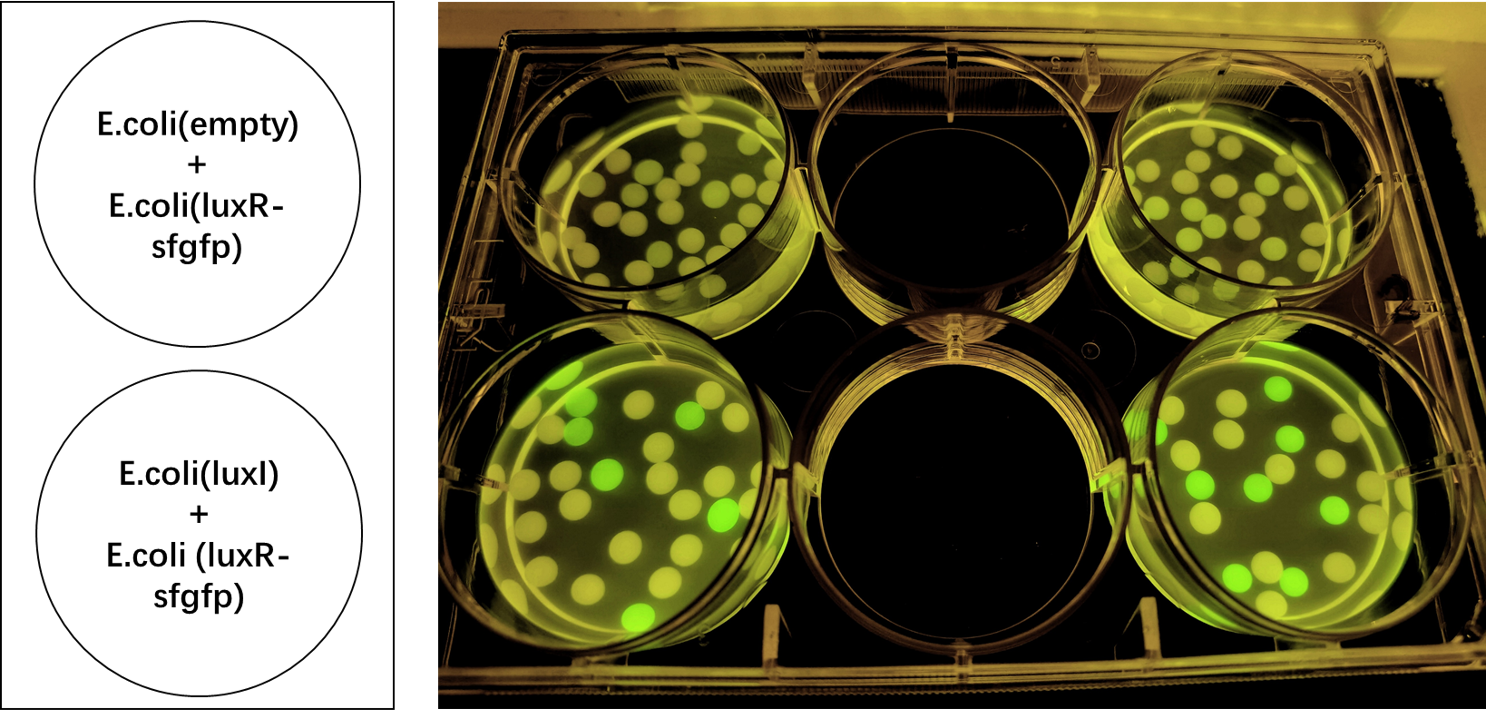
Proof of function
We encapsulated E. coli with this composite part and E. coli with LuxI into alginate gel beads. The E. coli with LuxI secreted the 3OC6 HSL signal molecule, and the E. coli with LuxR received the signal and regulate the expression of downstream sfGFP, making it express. So we can detect green fluorescence in the latter E. coli under the irradiation of ultraviolet light.
Quantitative analysis
Experiment approach
1. Dilute the E.coli DH5α cultures transformed with BBa_K4115039 that were stored at 4℃ 1:100 into 3ml LB medium and grow at 37℃ with shaking at 220rpm overnight.
2. Dilute the overnight cultures 1:100 into 3ml LB medium and grow at 37℃ with shaking at 220rpm for 3h. Measure their OD600 with Nanodrop One.
3. Dilute the culture with LB medium to OD600~0.1. Add 600ul diluted cultures to each 1.5ml tube. Add 0.6 ul autoinducer solution to each tube. The final concentrations of the autoinducer range from 10-4 to 10-14 M.
4. After culturing at 37℃ with shaking at 220rpm for three hours, add 200ul to each well of the black bottom 96-well plate for fluorescence intensity testing. Use an excitation wavelength of 485nm and an emission wavelength of 535nm.
5. Add 200ul to each well of the transparent 96-well plate for OD600 measurement.
Results
We process the data by dividing the fluorescence intensity by the OD600 and get Figure 2. Thus, we can conclude the response intensity of LuxR to 3O6C HSL at different concentrations.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 985
References
[1] Kylilis, N., Tuza, Z.A., Stan, GB. et al. Tools for engineering coordinated system behaviour in synthetic microbial consortia. Nat Commun 9, 2677 (2018). https://doi.org/10.1038/s41467-018-05046-2