Difference between revisions of "Part:BBa K4115039"
| Line 27: | Line 27: | ||
==Proof of function== | ==Proof of function== | ||
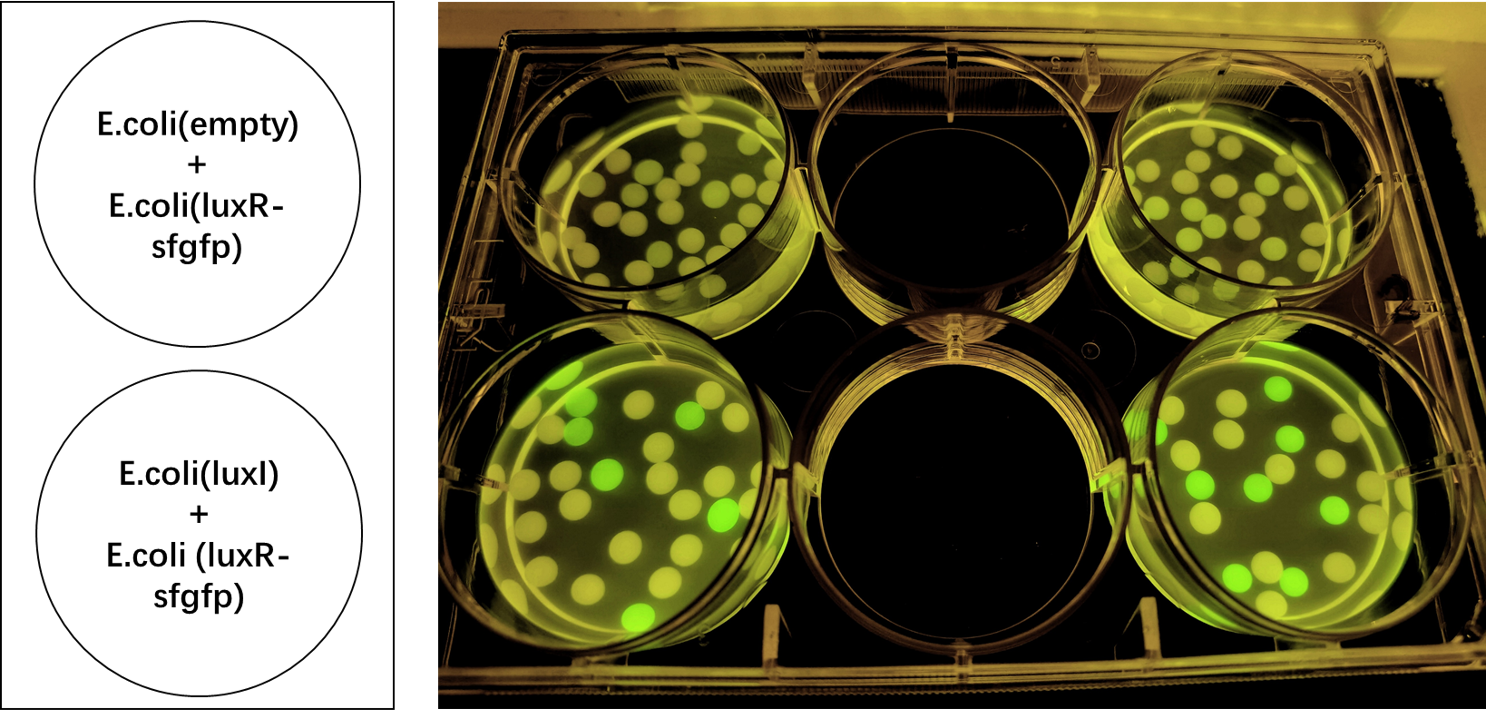
| − | We encapsulated E. coli with this composite part and <i>E. coli</i> with LuxI into alginate gel beads. The <i>E. coli</i> with LuxI secreted the 3OC6 HSL signal molecule, and the <i>E. coli</i> with LuxR received the signal and regulate the expression of downstream sfGFP, making it express. So we can detect green fluorescence in the latter <i>E. coli</i> under the irradiation of ultraviolet light. | + | We encapsulated <i>E. coli</i> with this composite part and <i>E. coli</i> with LuxI into alginate gel beads. The <i>E. coli</i> with LuxI secreted the 3OC6 HSL signal molecule, and the <i>E. coli</i> with LuxR received the signal and regulate the expression of downstream sfGFP, making it express. So we can detect green fluorescence in the latter <i>E. coli</i> under the irradiation of ultraviolet light. |
[[File:LuxR regulations between E.coli.jpg|500px|thumb|left|'''Figure 1:''' sfGFP is expressed under the regulation of LuxR between <i>E. coli</i> in a single gel bead. Two parallel groups are given and the above two are control groups.]]<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | [[File:LuxR regulations between E.coli.jpg|500px|thumb|left|'''Figure 1:''' sfGFP is expressed under the regulation of LuxR between <i>E. coli</i> in a single gel bead. Two parallel groups are given and the above two are control groups.]]<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
| Line 33: | Line 33: | ||
===Quantitative analysis=== | ===Quantitative analysis=== | ||
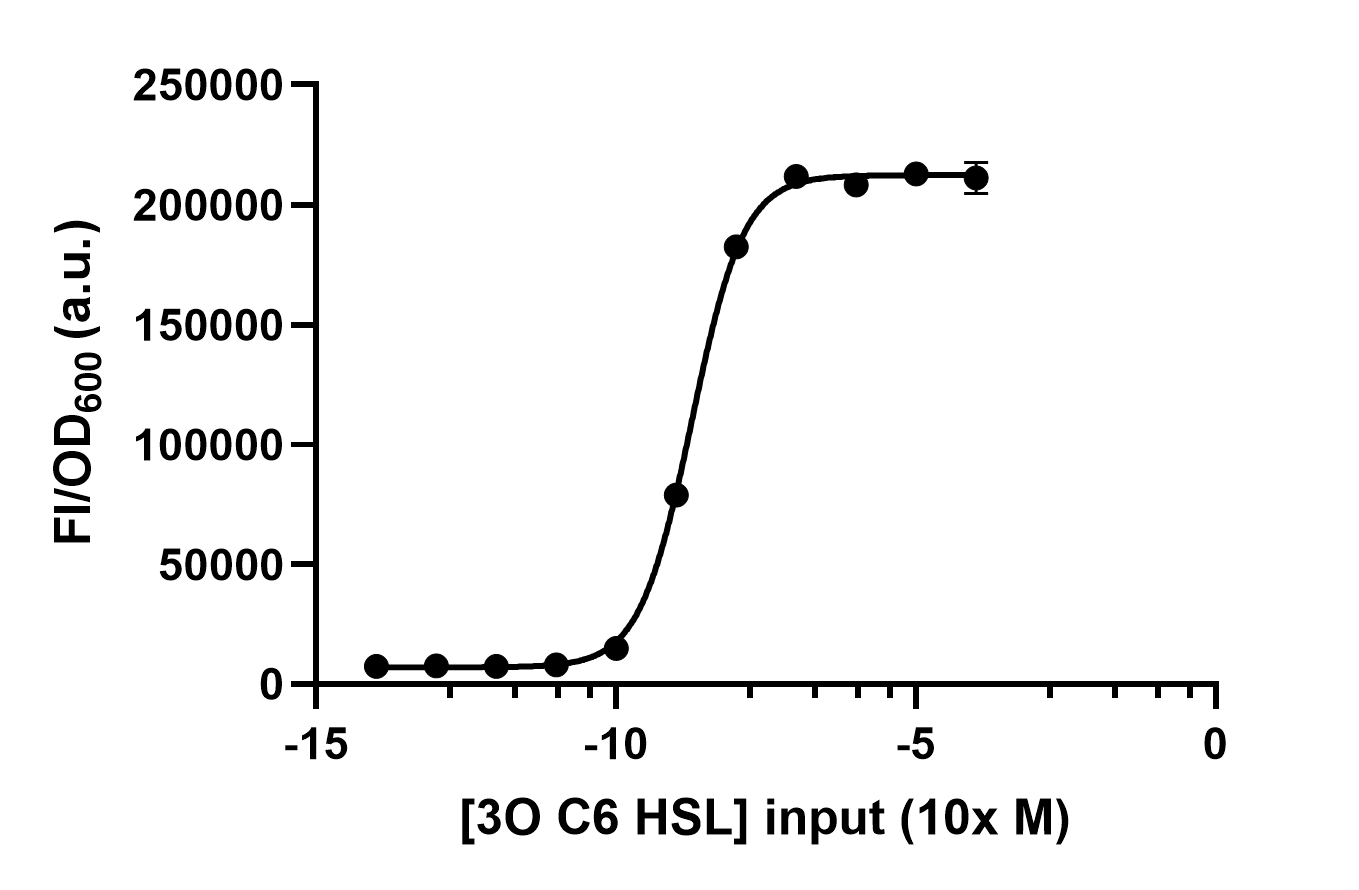
| − | We set | + | We set groups of <i>E. coli</i> with LuxR and add different concentrations of 3O6C HSL to test the response intensity of LuxR. We conclude the data by dividing the fluorescence intensity by the OD600 and get Figure 2. |
[[File:LuxR receive under different AHL concentrations input.jpg|500px|thumb|left|'''Figure 2:''' Different 3O6C HSL concentrations are inputed to test LuxR response intensity.]]<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | [[File:LuxR receive under different AHL concentrations input.jpg|500px|thumb|left|'''Figure 2:''' Different 3O6C HSL concentrations are inputed to test LuxR response intensity.]]<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
Revision as of 12:56, 4 October 2022
J23101-B0034-luxR-B0015-Lux pR-B0034-sfGFP-B0015
| J23101-B0034-luxR-B0015-Lux pR-B0034-sfGFP-B0015 | |
|---|---|
| Function | Gene regulator |
| Use in | E. coli |
| RFC standard | RFC 10, RFC 10 compatible |
| Backbone | pBBR1, pUC57 |
| Submitted by | ShanghaiTech_China |
This composite part is used to detect the response of LuxR (BBa_C0062) to AHL signaling molecules [1]. When LuxR receives the signal of AHL, it can regulate the downstream Lux pR (BBa_R0062) to make superfolder GFP (BBa_K4115000) express.
This part is togther used with LuxI (BBa_C0161), an AHL(3OC6 HSL here) sender.We can transform the plasmid into the A.caulinodans ORS571 to construct communications and regulations system between E. coli and S.elongatus HL7942 through quorum sensing signals.
Proof of function
We encapsulated E. coli with this composite part and E. coli with LuxI into alginate gel beads. The E. coli with LuxI secreted the 3OC6 HSL signal molecule, and the E. coli with LuxR received the signal and regulate the expression of downstream sfGFP, making it express. So we can detect green fluorescence in the latter E. coli under the irradiation of ultraviolet light.
Quantitative analysis
We set groups of E. coli with LuxR and add different concentrations of 3O6C HSL to test the response intensity of LuxR. We conclude the data by dividing the fluorescence intensity by the OD600 and get Figure 2.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 985
References
[1] Kylilis, N., Tuza, Z.A., Stan, GB. et al. Tools for engineering coordinated system behaviour in synthetic microbial consortia. Nat Commun 9, 2677 (2018). https://doi.org/10.1038/s41467-018-05046-2