Difference between revisions of "Part:BBa I746916"
| Line 35: | Line 35: | ||
</html>[[Image:T--Linkoping_Sweden--CBD-sfGFPstorodl4.jpeg|300px|thumb|left|<b><i>Figure 1.</i></b> 1 liter growth medium containing induced CBD-sfGFP BL21 (DE3) on a UV-table. ]] <html> | </html>[[Image:T--Linkoping_Sweden--CBD-sfGFPstorodl4.jpeg|300px|thumb|left|<b><i>Figure 1.</i></b> 1 liter growth medium containing induced CBD-sfGFP BL21 (DE3) on a UV-table. ]] <html> | ||
| − | <br> | + | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> |
The effect of IPTG on the protein expression of T7-CBD-sfGFP in E.coli BL21 (DE3) and Vibrio.natriegens Vmax was studied using an induction gradient with a range spanning from 0-1.5 mM IPTG. This was done by placing 200 µl of the bacteria in a 96-well plate reader containing varying IPTG concentration levels. Each IPTG concentration contained replicates of 4, and was measured over 45 hours. The resuts can be seen below in Figure t | The effect of IPTG on the protein expression of T7-CBD-sfGFP in E.coli BL21 (DE3) and Vibrio.natriegens Vmax was studied using an induction gradient with a range spanning from 0-1.5 mM IPTG. This was done by placing 200 µl of the bacteria in a 96-well plate reader containing varying IPTG concentration levels. Each IPTG concentration contained replicates of 4, and was measured over 45 hours. The resuts can be seen below in Figure t | ||
| − | + | ||
| − | + | ||
<br><br> | <br><br> | ||
<br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br> | ||
Revision as of 11:38, 12 October 2019
superfolder GFP coding sequence
This is the coding sequence of superfolder GFP (Pedelacq et al (2006): "Engineering and characterization of a superfolder green fluorescent protein", Nature Biotech 24 (1) January 2006).
More information about the properties of this GFP in relation to the currently used mut3GFP can be found at: http://openwetware.org/wiki/IGEM:Cambridge/2008/Improved_GFP.
It carries the following amino acid changes with respect to mut3 GFP (E0040), the currently most commonly used GFP in the registry:
S30R, Y39N, F64L, G65T, F99S, N105T, Y145F, M153T, V163A, I171V, A206V
Its in-vivo properties are considerably improved with respect to mut3 - it develops fluorescence about 3fold faster than mut3 GFP and reaches 4fold higher absolute fluorescence levels. Fluorescenct colonies can be identified with the naked eye even without UV or blue light illumination (that is to say the amount of blue light in normal daylight or lablight is sufficient). Additionally it is more stable in vitro and refolds faster after in vitro denaturation with respect to mut3 GFP.
Note: Superfolder GFP is available in constructs driven by the pBAD and T7 promoters: part numbers I746908 and I746909 respectively. Additionally 6-his tagged versions for protein purification exist: I746914 (pBAD driven) and I746915 (T7 driven).
Contribution
Group: Linkoping_Sweden iGEM 2019
Author: Andreas Holmqvist and Leo Juhlin
Summary: We studied the a bacteria culture of E.coli BL21(DE3) containing CBD-asPink protein with a constitutive promotor to see if the protein could be used for a pink/white screening. We also tested the bindning capacity of the CBD-asPink protein to a micro cellulose bandage and then studied the release mechanism.
Documentation:
- the BBa_B0034 ribosome binding site
- the BBa_J23110 Constitutive promotor
- BBa_K2656004: the J23106 promoter in its Golden Braid compatible version from our [http://2018.igem.org/Team:Valencia_UPV/Part_Collection Part Collection]
- BBa_K2656009: the B0030 ribosome biding site in its Golden Braid compatible version from our [http://2018.igem.org/Team:Valencia_UPV/Part_Collection Part Collection]
- BBa_K2656013: This part.
- BBa_K2656026: the B0015 transcriptional terminator in its Golden Braid compatible version from our [http://2018.igem.org/Team:Valencia_UPV/Part_Collection Part Collection]
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 13
To verify the fluorescence of CBD-sfGFP, e.Coli BL21 (DE3) containing CBD-sfGFp was grown in 1 liter growth medium with 25 µg/ml chloramphenicol and induced with 1 mM Isopropyl β-d-1-thiogalactopyranoside (IPTG). The Erlenmayer flask was placed on a UV-table emitting 302 nm which can se been below, Figure y . The picture shows that an induced culture of CBD-sfGFP has the ability to flourescence at 302 nm.
The effect of IPTG on the protein expression of T7-CBD-sfGFP in E.coli BL21 (DE3) and Vibrio.natriegens Vmax was studied using an induction gradient with a range spanning from 0-1.5 mM IPTG. This was done by placing 200 µl of the bacteria in a 96-well plate reader containing varying IPTG concentration levels. Each IPTG concentration contained replicates of 4, and was measured over 45 hours. The resuts can be seen below in Figure t
Group: Valencia_UPV iGEM 2018
Author: Adrián Requena Gutiérrez
Summary: We have adapted the part to be able to assemble transcriptional units with the Golden Gate method and we have done the characterization of this protein.
Documentation:
We adapted the CDS BBa_I746916 to be used to assemble composite parts using the Golden Gate method creating [https://parts.igem.org/Part:BBa_K2656013 BBa_K2656013]. Then we made the characterization of the part: The characterization of this protein (and by extension of all the other part that codify for the sfGFP) was performed with our transcriptional unit [https://parts.igem.org/Part:BBa_K2656101 BBa_K2656101]. This transcriptional unit was assembled in a [http://2018.igem.org/Team:Valencia_UPV/Design Golden Braid alpha 1 plasmid] including the following parts:
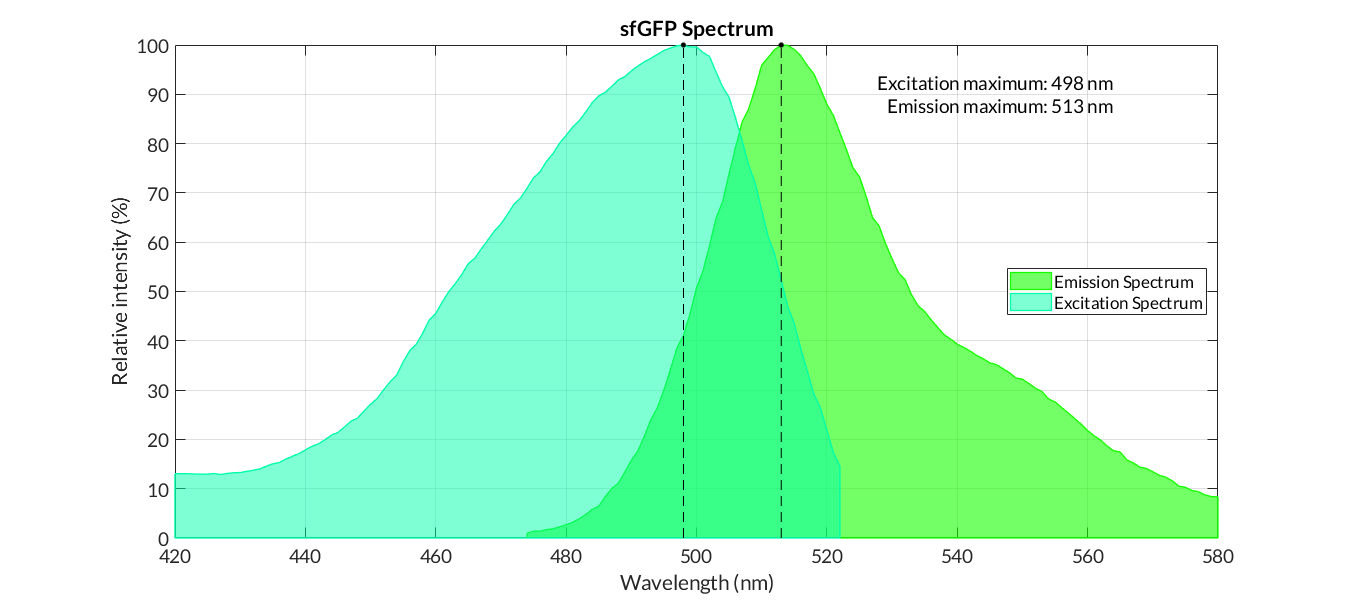
In order to carry out a correct characterization of the protein and to be able to use it to make measurements of the different transcriptional units that we have assembled with it, we have obtained the emission and excitation spectra in the conditions of our equipment. By using this [http://2018.igem.org/Team:Valencia_UPV/Experiments#spectra protocol] with the parameters of Table 1, Figure 1 has been obtained.
| Table 1. Parameters used to obtain the spectra | |||
| Parameter | Value | ||
| Number of samples | 6 | ||
| Excitation Wavelength measurement range (nm) | [420-525] | ||
| Emission wavelenght (nm) | 545 | ||
| Emission Wavelength measurement range (nm) | [470-580] | ||
| Excitation wavelenght (nm) | 450 | ||
| Gain (G) | 50 | ||
Improvement
Group: iGEM2018_Jilin_China
Author:Zihao Wang
Summary: This year our team registered the superfolder GFP designed by Overkamp W et al with a BBa_K2541400 (sfGFP_optimism, https://parts.igem.org/Part:BBa_K2541400). Compared with superfolder GFP(BBa_I746916), sfGFP_optimism (BBa_K2541400) is BbsI restriction site free, so it can be used in GoldenGate assembly to achieve efficient and rapid assembly of gene fragments.
Document:
1. Usage and Biology
Green fluorescent protein (GFP) exhibits intrinsic fluorescence and is commonly used as a reporter gene in intact cells and organisms [1]. Many mutants of the protein with either modified spectral properties, increased fluorescence intensity, or improved folding properties have been reported [2].
GFP often misfolds when expressed as fusions with other proteins, while a robustly folded version of GFP, called superfolder GFP (sfGFP), was developed and described by Pédelacq et al at 2006[3] that folds well even when fused to poorly folded polypeptides. We decided to use sfGFP as our reporter protein due to its faster folded feature and higher fluorescence intensity. The superfolder GFP had been registered in iGEM BBa_I746916. There is another superfolder GFP designed by Overkamp W et al at 2013[4], which is a codon optimized sfGFP. It was be used in Escherichia coli by Segall-Shapiro T H et al at 2018[5].
This year our team registered the superfolder GFP designed by Overkamp W et al with a BBa_K2541400 (called sfGFP_optimism) and BBa_K2541401(called sfGFP(BbsI free)).
Compared with superfolder GFP (BBa_I746916), sfGFP_optimism (BBa_K2541400) and sfGFP(BbsI free)(BBa_K2541401) are BbsI restriction site free, and the BbsI restriction endonuclease is an economical and efficient enzyme used in Golden Gate assembly, so sfGFP_optimism and sfGFP(BbsI free) can be used in Golden Gate assembly to achieve efficient and rapid assembly of gene fragments.
Figure 1. Expression of three types of sfGFP(BBa_I746916, BBa_K2541401, BBa_K2541400), cultivated overnight.
2. Characterization
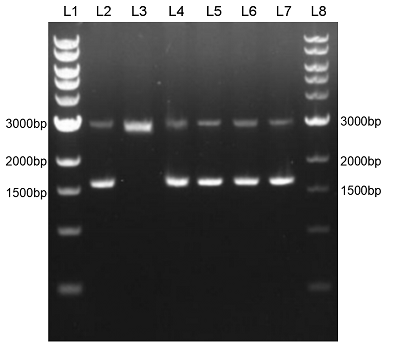
We constructed sfGFP_optimism (BBa_K2541400) sequence and superfolder GFP (BBa_I746916) on the pSB1C3 vector. The BbsI recognition site free was confirmed by nucleic acid electrophoresis (Figure 2). The sfGFP_optimism can not be digested by BbsI. And the length of these sequences are correct.
Figure 2. L1: 1kb DNA marker; L2: BBa_I746916; L3: BBa_I746916+BbsI; L4: BBa_K2541401; L5: BBa_K2541401+BbsI; L6: BBa_K2541400; L7: BBa_K2541400+BbsI; L8: 1kb DNA marker.
We got the emission and excitation spectra of two type sfGFP: sfGFP_optimism (BBa_K2541400), sfGFP(BbsI free)(BBa_K2541401) and superfolder GFP (BBa_I746916) (Figure 3).



Figure 3. Emission and Excitation Spectra of sfGFP_optimism(BBa_K2541400) and sfGFP(BBa_I746916)
For further characterization, we detected the expression intensity of these two types of sfGFP. According to the results (Figure 4), we found out that the fluorescence intensity of sfGFP_optimism (BBa_K2541400) is nearly 2.6 times higher than superfolder GFP (BBa_I746916).

Figure 5. The expression of three types of sfGFP in E.coli. The grey line represents fluorescent expression of sfGFP_optimism (BBa_K2541400), the orange line represents fluorescent expression of sfGFP(BbsI free) (BBa_K2541401), the blue line represents fluorescent expression of superfolder GFP (BBa_I746916) and the yellow line represents fluorescent expression of negative control (BBa_J364007).
3. Conclusion
We have made an improvement on the superfolder GFP (BBa_I746916). Our sfGFP_optimism (BBa_K2541400) is BbsI restriction site free, which can be used in Golden Gate assembly to achieve efficient and rapid assembly of gene fragments. And its fluorescence intensity is higher than superfolder GFP (BBa_I746916).
Sequence and Features