Difference between revisions of "Featured Parts:Light Sensor"
| (One intermediate revision by one other user not shown) | |||
| Line 3: | Line 3: | ||
From Levskyaya ''et al.'' | From Levskyaya ''et al.'' | ||
| − | '''We have designed a bacterial system that is switched between different states by red light. The system consists of a synthetic sensor kinase that allows a lawn of bacteria to function as a biological film, such that the projection of a pattern of light on to the bacteria produces a high-definition (about 100 megapixels per square inch), two-dimensional chemical image.''' | + | {| |
| + | | width=25px | | ||
| + | |'''"We have designed a bacterial system that is switched between different states by red light. The system consists of a synthetic sensor kinase that allows a lawn of bacteria to function as a biological film, such that the projection of a pattern of light on to the bacteria produces a high-definition (about 100 megapixels per square inch), two-dimensional chemical image."''' | ||
| + | |} | ||
===Sample photos=== | ===Sample photos=== | ||
| Line 33: | Line 36: | ||
[[Image:Coliroidparts.jpg]]<br> | [[Image:Coliroidparts.jpg]]<br> | ||
Diagram courtesy of [http://openwetware.org/wiki/Drew_Endy Drew Endy]. | Diagram courtesy of [http://openwetware.org/wiki/Drew_Endy Drew Endy]. | ||
| + | |||
| + | For details on system implementation, see the reference at the bottom of this page. | ||
===BioBricks parts list=== | ===BioBricks parts list=== | ||
| Line 48: | Line 53: | ||
====Related parts==== | ====Related parts==== | ||
*[https://parts.igem.org/wiki/index.php/Part:BBa_M30109 BBa_M30109] | *[https://parts.igem.org/wiki/index.php/Part:BBa_M30109 BBa_M30109] | ||
| + | |||
| + | ====Some interesting Results=== | ||
| + | We, UNIST_KOREA (2011 iGEM) team obtained the plasmids encoding the hybrid light receptor, biosynthesis pathway for PCB production and Pompc expressing cI from Christ Voigt. | ||
| + | |||
| + | '''Characterization of Light receptor and its invertor''' | ||
| + | |||
| + | Orginally all light regulated promoters repress gene expression in the presence of light. However, for our experiment we need to switch on the expression of the target gene in the presence of light. Hence, we tried to engineer an invertor such that light favors the expression of the target gene. The invertor we exploited was the famous lambda repressor, cI. | ||
| + | |||
| + | In order to avoid the leaky expression of cI in the presence of light and enhance the expression of the target gene, we integrated the Pompc expressing cI into the chromosome of ''E. coli''. We tried to regulate the expression of target gene by light mediated modulation of the level of chromosomally encoded cI (Fig 1). | ||
| + | |||
| + | [[Image:SKL9.jpg|500px]] | ||
| + | |||
| + | '''Effect of duration of light and age of cells on gene expression''' | ||
| + | |||
| + | We also wanted to characterize the duration of light needed to induce gene expression. Our results indicate that it takes almost 1 hour for the PompC promoter to be inactivated, cI being degraded and the target gene being expressed. The 1 hour duration was irrespective of the stage in which the cells were induced i.e. even when the cells were induced in log phase or in stationary phase, it takes 1 hour for the target gene to be expressed. However, fold change in gene expression differs with the phase in which gene expression was induced (Fig 2). | ||
| + | |||
| + | Figure 2 | ||
| + | [[Image:SKL5.jpg|500px]] | ||
| + | |||
| + | '''Cross talk between Cellular function and the Light receptor''' | ||
| + | |||
| + | The light receptor was a hybrid of the cyanobacterial light receptor and ''E. coli'' osmo regulator. Further, the promoter regulated by light was originally regulated by osmolality i.e. higher concentration of salt/sugar would repress the promoter. We have deleted the native EnvZ gene in order to avoid the cellular cross talk. However, despite the deletion of envZ gene, promoter Pompc was regulated by osmolarity (Figure 3). This indicates the presence of a cross-talk between the native cellular function and the light receptor. | ||
| + | |||
| + | [[Image:SKL10.jpg|700px]] | ||
| + | |||
| + | |||
| + | '''Disadvantage''' | ||
| + | The crosstalk observed in the light receptor would be a disadvantageous feature in case light mediated gene expression systems are to be used in industrial process were the osmolarity effects will be high. | ||
| + | |||
| + | |||
==Contact== | ==Contact== | ||
| Line 57: | Line 92: | ||
===Other=== | ===Other=== | ||
| − | + | #[http://openwetware.org/wiki/BE.109:Systems_engineering MIT Biological Engineering laboratory module] on bacterial photography developed by [http://openwetware.org/wiki/Natalie_Kuldell Natalie Kuldell] | |
| − | + | #[http://openwetware.org/wiki/LightCannon Instructions] for how to build a "light cannon" for use in bacterial photography. | |
Latest revision as of 02:14, 6 October 2011
Contents
System overview
From Levskyaya et al.
| "We have designed a bacterial system that is switched between different states by red light. The system consists of a synthetic sensor kinase that allows a lawn of bacteria to function as a biological film, such that the projection of a pattern of light on to the bacteria produces a high-definition (about 100 megapixels per square inch), two-dimensional chemical image." |
Sample photos
Here are a selection of sample coliroid taken with the bacterial photography system.
| http://openwetware.org/images/9/9a/Macintosh_HD-Users-nkuldell-Desktop-bacterialselfportrait.jpg | http://openwetware.org/images/8/83/UT_HelloWorld.jpg | http://openwetware.org/images/thumb/d/d1/ColiroidEllington.png/200px-ColiroidEllington.png | http://openwetware.org/images/6/68/ColiroidFSM.jpg |
| [http://openwetware.org/wiki/Jeff_Tabor Jeff Tabor] holding a coliroid. Photo credit: Marsha Miller, University of Texas at Austin. Image courtesy of UT/UCSF. |
Hello World coliroid published in Levskaya et al., Nature, 2005. | This is a coliroid portait of Andy Ellington. You can compare it with the [http://www.icmb.utexas.edu/images/faculty/ellington.jpg real Andy]. Image courtesy of UT/UCSF. | This is a coliroid of the [http://venganza.org Flying Spaghetti Monster]. Image courtesy of UT/UCSF. |
Device implementation
This system consists of two devices.
- A light sensor which takes red light as an input and produces PoPS as an output.
- A color generator which takes a PoPS signal as input and generate color as an output.
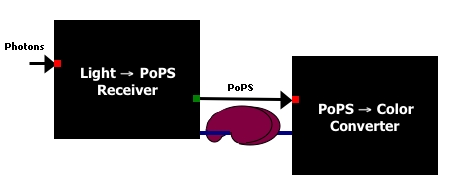
Diagram courtesy of [http://openwetware.org/wiki/Drew_Endy Drew Endy].
Parts implementation
This diagram shows the list of parts that make up each of the two devices.
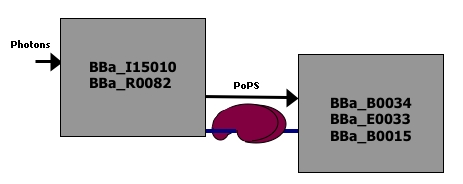
Diagram courtesy of [http://openwetware.org/wiki/Drew_Endy Drew Endy].
For details on system implementation, see the reference at the bottom of this page.
BioBricks parts list
Light sensing parts
Color generating parts
Related parts
=Some interesting Results
We, UNIST_KOREA (2011 iGEM) team obtained the plasmids encoding the hybrid light receptor, biosynthesis pathway for PCB production and Pompc expressing cI from Christ Voigt.
Characterization of Light receptor and its invertor
Orginally all light regulated promoters repress gene expression in the presence of light. However, for our experiment we need to switch on the expression of the target gene in the presence of light. Hence, we tried to engineer an invertor such that light favors the expression of the target gene. The invertor we exploited was the famous lambda repressor, cI.
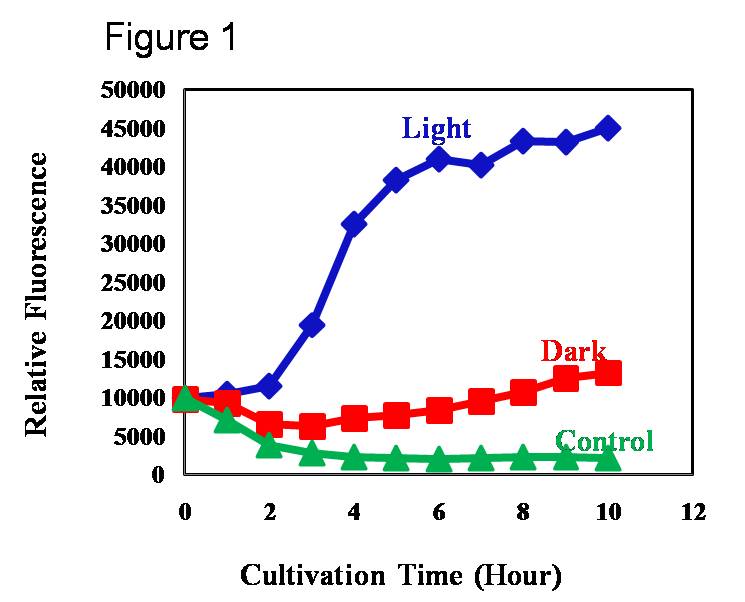
In order to avoid the leaky expression of cI in the presence of light and enhance the expression of the target gene, we integrated the Pompc expressing cI into the chromosome of E. coli. We tried to regulate the expression of target gene by light mediated modulation of the level of chromosomally encoded cI (Fig 1).
Effect of duration of light and age of cells on gene expression
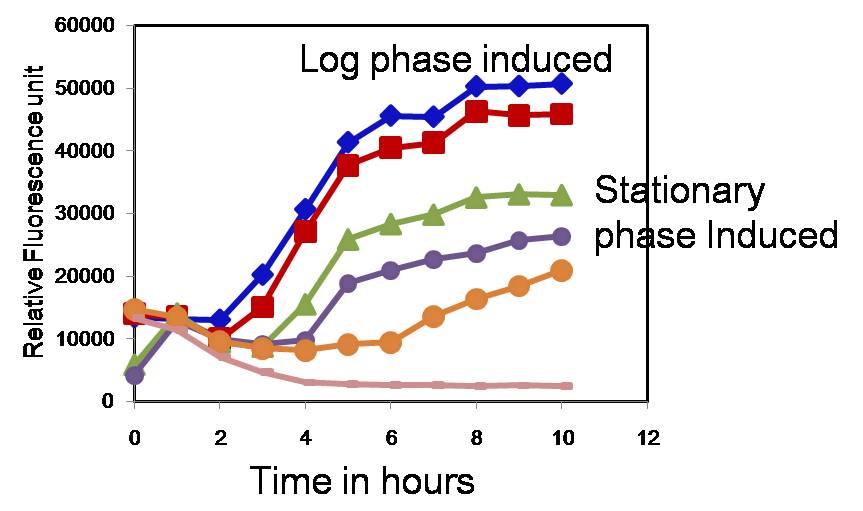
We also wanted to characterize the duration of light needed to induce gene expression. Our results indicate that it takes almost 1 hour for the PompC promoter to be inactivated, cI being degraded and the target gene being expressed. The 1 hour duration was irrespective of the stage in which the cells were induced i.e. even when the cells were induced in log phase or in stationary phase, it takes 1 hour for the target gene to be expressed. However, fold change in gene expression differs with the phase in which gene expression was induced (Fig 2).
Cross talk between Cellular function and the Light receptor
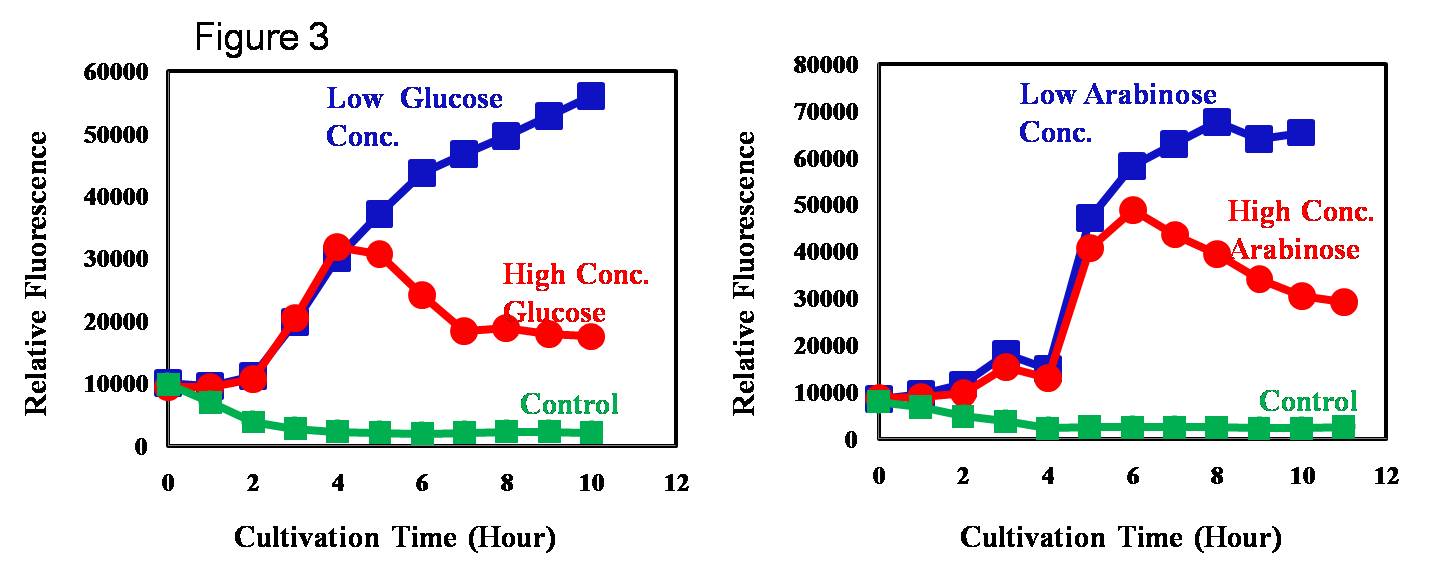
The light receptor was a hybrid of the cyanobacterial light receptor and E. coli osmo regulator. Further, the promoter regulated by light was originally regulated by osmolality i.e. higher concentration of salt/sugar would repress the promoter. We have deleted the native EnvZ gene in order to avoid the cellular cross talk. However, despite the deletion of envZ gene, promoter Pompc was regulated by osmolarity (Figure 3). This indicates the presence of a cross-talk between the native cellular function and the light receptor.
Disadvantage
The crosstalk observed in the light receptor would be a disadvantageous feature in case light mediated gene expression systems are to be used in industrial process were the osmolarity effects will be high.
Contact
- System design and implementation by [http://openwetware.org/wiki/User:Levskaya Anselm Levskaya], Aaron Chevalier, [http://openwetware.org/wiki/Jeff_Tabor Jeff Tabor], [http://openwetware.org/wiki/User:LLavery Laura Lavery], Matthew Levy, Eric Davidson, Alexander Scouras, [http://ellingtonlab.org/ Andy Ellington], Ed Marcotte, and [http://www.voigtlab.ucsf.edu/ Chris Voigt].
References
Papers
- Engineering Escherichia coli to see light
Nature 24 November 2005 DOI:10.1038/nature04405
A. Levskaya et al.
[http://www.nature.com/nature/journal/v438/n7067/full/nature04405.html URL] [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16306980 Pubmed] [http://www.hubmed.org/display.cgi?uids=16306980 Hubmed]
Other
- [http://openwetware.org/wiki/BE.109:Systems_engineering MIT Biological Engineering laboratory module] on bacterial photography developed by [http://openwetware.org/wiki/Natalie_Kuldell Natalie Kuldell]
- [http://openwetware.org/wiki/LightCannon Instructions] for how to build a "light cannon" for use in bacterial photography.