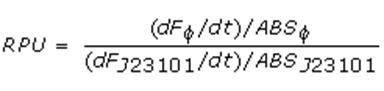Difference between revisions of "Part:BBa K173005:Experience"
(→Characterization) |
|||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
| − | |||
| − | |||
===Applications of BBa_K173005=== | ===Applications of BBa_K173005=== | ||
| + | |||
| + | |||
| + | ===BBa_K173005/BBa_Q04400 - tetR QPI - UNIPV-Pavia (Test performed by L. Pasotti, S. Zucca)=== | ||
| + | |||
| + | ====Description==== | ||
| + | |||
| + | This device takes PoPS as input and gives a PoPS output according to the transfer function of a logic inverter: tetR (BBa_C0040) is a repressor for tetR promoter (BBa_R0040), so high input signals give a low expression of tetR promoter and low input signals give a high expression of the promoter. | ||
| + | We have characterized <partinfo>BBa_K173005</partinfo> part (twin of <partinfo>BBa_Q04400</partinfo>) as a constitutive device, equivalent to tetR promoter, and as a genetic logic inverter. | ||
| + | <partinfo>BBa_K173005</partinfo> has been studied as constitutive device using <partinfo>BBa_K173008</partinfo>, which has been induced with different aTc concentrations in order to see if GFP levels changed as a function of aTc concentration. | ||
| + | On the other hand, <partinfo>BBa_K173005</partinfo> has been studied as a logic inverter by using <partinfo>BBa_K173008</partinfo>, <partinfo>BBa_K173009</partinfo> and <partinfo>BBa_K173026</partinfo>, in which tetR expression is driven by constitutive promoters of different and measured strengths (no promoter, <partinfo>BBa_J23100</partinfo> and <partinfo>BBa_J23118</partinfo> respectively). | ||
| + | |||
| + | [[Image:pv_A4_description.png]] | ||
| + | |||
| + | ===Characterization=== | ||
| + | Compatibility: E. coli TOP10 in pSB1A2 | ||
| + | |||
| + | This part has been characterized only in M9 supplemented medium. In LB only a measure in absence of inducer has been performed. | ||
| + | <html> | ||
| + | <table border=1 align="center" cellpadding=5px> | ||
| + | |||
| + | <tr> | ||
| + | <td rowspan=2 valign="bottom" align="center"><b>aTc concentration<br>[ng/ml]</b></td> | ||
| + | <td colspan=2 align="center"><b>LB</b></td> | ||
| + | <td colspan=2 align="center"><b>M9 supplemented</b></td> | ||
| + | </tr> | ||
| + | |||
| + | <tr> | ||
| + | |||
| + | <td><b>Doubling time [minutes]</b></td> | ||
| + | <td><b>RPU</b></td> | ||
| + | <td><b>Doubling time [minutes]</b></td> | ||
| + | <td><b>RPU</b></td> | ||
| + | </tr> | ||
| + | <tr align="center"> | ||
| + | <td> 0</td><td> 32 </td> | ||
| + | <td> 1.58 [ 1.52 ; 1.67] </td> | ||
| + | <td> 78 </td> <td> 1.36 [ 1.22 ; 1.65]</td> | ||
| + | </tr><tr align="center"> | ||
| + | <td> 25</td><td> not performed </td> | ||
| + | <td> not performed</td> | ||
| + | <td> 68 </td> <td> 1.62 [ 1.49 ; 1.97]</td> | ||
| + | </tr><tr align="center"> | ||
| + | <td> 50</td><td> not performed </td> | ||
| + | <td> not performed</td> | ||
| + | <td> 69 </td> <td> 1.59 [ 1.36 ; 2.10]</td> | ||
| + | </tr><tr align="center"> | ||
| + | <td> 75</td><td> not performed </td> | ||
| + | <td> not performed</td> | ||
| + | <td> 75 </td> <td> 1.59 [ 1.32 ; 2.03]</td> | ||
| + | </tr><tr align="center"> | ||
| + | <td> 100</td><td> not performed </td> | ||
| + | <td> not performed</td> | ||
| + | <td> 78 </td> <td> 1.75 [ 1.57 ; 2.10]</td> | ||
| + | </tr><tr align="center"> | ||
| + | <td> 200</td><td> not performed </td> | ||
| + | <td> not performed</td> | ||
| + | <td> 82 </td> <td> 1.57 [ 1.35 ; 1.98]</td> | ||
| + | </tr><tr align="center"> | ||
| + | <td> 300</td><td> not performed </td> | ||
| + | <td> not performed</td> | ||
| + | <td> 85 </td> <td> 1.31 [ 1.21 ; 1.51]</td> | ||
| + | </tr> | ||
| + | |||
| + | </table> | ||
| + | </html> | ||
| + | |||
| + | |||
| + | {|align="center" | ||
| + | |[[Image:PV_A8_M9.png|700px|thumb|<partinfo>BBa_K173008</partinfo> Growth curves for BBa_K173008 in M9]] | ||
| + | |- | ||
| + | |[[Image:PV_A4_Scell_M9.png|700px|thumb|<partinfo>BBa_K173008</partinfo>(dGFP/dt)/O.D. in M9]] | ||
| + | |- | ||
| + | |[[Image:PV_A4_IndCurvM9.png|700px|thumb|<partinfo>BBa_K173008</partinfo> Induction curve of BBa_K173008 in M9]] | ||
| + | |- | ||
| + | |[[Image:PV_A4_LB_log_inv.png|700px|thumb|<partinfo>BBa_K173008</partinfo>Logical inverter: RPU of BBa_K173008 in function of RPU of promoter used for tetR expression in LB]] | ||
| + | |} | ||
| + | |||
| + | The last image reports the inverter behaviour of tetR QPI component in LB medium. Considering RPU of promoter regulating tetR expression as input and RPU of tetR QPI device as output it is possible to characterize the logic inverter reported in figure. NB: RPU of promoters <partinfo>BBa_J23100</partinfo> and <partinfo>BBa_J23118</partinfo> have been calculated as reported in the <partinfo>BBa_J23100</partinfo> Experience page, while the absence of promoter has been associated to RPU=0. When input (RPU of the promoter expressing tetR) is high, the output is low and the behavior is opposite for high input RPU. | ||
| + | |||
| + | =====Conclusions===== | ||
| + | The expected behaviour of this part was not to be affected by the presence of inducer, because the repressor should not be present. In fact, tetR production isn't regulated by any promoter and Ptet can work as a constitutive one. | ||
| + | |||
| + | Actually, the activity of promoter is not independent from aTc concentration, probably because of spurious transcription of tetR repressor in the high copy number plasmid. The experiments concerning this part have been performed in M9 supplemented medium. In LB medium the component has been tested only in absence of inducer and this has been useful to estimate three points of the transfer function of <partinfo>BBa_K173005</partinfo> as a logic inverter. | ||
| + | |||
| + | We can say that the behaviour of <partinfo>BBa_K173005</partinfo> as a logic inverter has been validated, even if more points of the transfer function have to be measured. | ||
| + | |||
| + | |||
| + | <div align="right"> | ||
| + | [[#top|Top]] | ||
| + | </div> | ||
| + | |||
| + | ==== '''Growth conditions''' ==== | ||
| + | =====Microplate reader experiments===== | ||
| + | *8 ul of long term storage glycerol stock were inoculated in 5 ml of LB + suitable antibiotic in a 15 ml falcon tube and incubated at 37°C, 220 rpm for about 16 hours. | ||
| + | *The grown cultures were then diluted 1:100 in 5 ml of LB or M9 supplemented medium and incubated in the same conditions as before for about 4 hours. | ||
| + | *These new cultures were diluted to an O.D.600 of 0.02 (measured with a TECAN F200 microplate reader on a 200 ul of volume per well; it is not comparable with the 1 cm pathlength cuvette) in a sufficient amount of medium to fill all the desired microplate wells. | ||
| + | *These new dilutions were aliquoted in a flat-bottom 96-well microplate, avoiding to perform dynamic experiments in the microplate frame (see [http://2009.igem.org/Team:UNIPV-Pavia/Methods_Materials/Evaporation Frame effect section] for details). All the wells were filled with a 200 ul volume. | ||
| + | *If required, 2 ul of inducer were added to each single well. | ||
| + | *The microplate was incubated in the Tecan Infinite F200 microplate reader and fluorescence (when required) and absorbance were measured with this automatic protocol: | ||
| + | **37°C constant for all the experiment; | ||
| + | **sampling time of 5 minutes; | ||
| + | **fluorescence gain of 50; | ||
| + | **O.D. filter was 600 nm; | ||
| + | **GFP filters were 485nm (ex) / 540nm (em); | ||
| + | **15 seconds of linear shaking (3mm amplitude) followed by 10 seconds of waiting before the measurements in order to make a homogeneous culture. | ||
| + | **Variable experiment duration time (from 3 to 24 hours). | ||
| + | |||
| + | ==== '''Data analysis''' ==== | ||
| + | ====Growth curves==== | ||
| + | All our growth curves have been obtained subtracting for each time sample the broth O.D.600 measurement from that of the culture; broth was considered in the same conditions of the culture (e.g. induced with the same inducer concentration). | ||
| + | |||
| + | =====Doubling time===== | ||
| + | The natural logarithm of the growth curves (processed according to the above section) was computed and the linear phase (corresponding to the bacterial exponential growth phase) was isolated by visual inspection. Then the linear regression was performed in order to estimate the slope of the line ''m''. Finally the doubling time was estimated as ''d''=ln(2)/''m'' [minutes]. | ||
| + | |||
| + | In the case of multiple growth curves for a strain, the mean value of the processed curves was computed for each time sample before applying the above described procedure. | ||
| + | |||
| + | =====Relative Promoter Units (RPUs)===== | ||
| + | The RPUs are standard units proposed by Kelly J. et al., 2008, in which the transcriptional strength of a promoter can be measured using a reference standard, just like the ground in electric circuits. | ||
| + | |||
| + | RPUs have been computed as: | ||
| + | |||
| + | [[Image:pv_rpu_formula.jpg|230px]] | ||
| + | |||
| + | in which: | ||
| + | *phi is the considered promoter and J23101 is the reference standard promoter (taken from Anderson Promoter Collection); | ||
| + | *F is the blanked fluorescence of the culture, computed subtracting for each time sample fluorescence measure for negative control from that of culture, where the negative control is a non-fluorescent strain (in our experiment it is usually used TOP10 strain bearing <partinfo>BBa_B0032</partinfo> or <partinfo>BBa_B0033</partinfo>, which are symmply RBSs do not have expression systems for reporter genes); | ||
| + | *ABS is the blanked absorbance (O.D.600) of the culture, computed as described in "Growth curves" section. | ||
| + | |||
| + | RPU measurement has the following advantages (under suitable conditions) | ||
| + | *it is proportional to PoPS (Polymerase Per Second), a very important parameter that expresses the transcription rate of a promoter; | ||
| + | *it uses a reference standard and so measurements can be compared between different laboratories. | ||
| + | |||
| + | The hypotheses on which RPU theory is based can be found in Kelly J. et al., 2008, as well as all the mathematical steps. From our point of view, the main hypotheses that have to be satisfied are the following: | ||
| + | *the reporter protein must have a half life higher than the experiment duration (we use GFPmut3, <partinfo>BBa_E0240</partinfo>, which has an estimated half life of at least 24 hours, and the experiments duration is always less than 7 hours); | ||
| + | *strain, plasmid copy number, antibiotic, growth medium, growth conditions, protein generator assembled downstream of the promoter must be the same in the promoter of interest and in J23101 reference standard. | ||
| + | *steady state must be valid, so (dF/dt)/ABS (proportional to the GFP synthesis rate per cell) must be constant. | ||
| + | |||
| + | ====Inducible systems==== | ||
| + | Every experiment is performed on the following cultures: | ||
| + | |||
| + | *the culture of interest (system studied expressing GFP) | ||
| + | *the benchmarck used to evaluate R.P.U. (<partinfo>BBa_K173001</partinfo> measurement part, that is <partinfo>BBa_J23101</partinfo> with <partinfo>BBa_E0240</partinfo> downstream) | ||
| + | *a negative control (generally, <partinfo>BBa_B0033</partinfo> RBS) | ||
| + | |||
| + | For inducible systems several plots are reported. The first plot is a panel containing 4 subplots, numerated this way: | ||
| + | {| | ||
| + | |(1) | ||
| + | |(2) | ||
| + | |- | ||
| + | |(3) | ||
| + | |} | ||
| + | |||
| + | Plot (1) contains growth curves of the cultures, after blank value has been removed. Every curve is calculated averaging on three replicates of the same culture and subtracting the blank for each time sample. Blank is calculated averaging the replicates of blank wells. | ||
| + | |||
| + | Plot (2) shows the logarithm of absorbance in exponential phase of bacterial growth, determined by a visual inspection of log-plots. These values are used to evaluate doubling time and R.P.U.. | ||
| + | |||
| + | Plot (3) contains (dGFP/dt)/O.D., the value named S_cell in Kelly J. et al., 2008 procedure for RPU evaluation. | ||
| + | |||
| + | In these plots are reported black veritcal lines that define the range of values used to evaluate RPU. It is important to underline, as explained in next paragraph, that RPU are calculated on cultures at the same O.D. level, not at the same time. | ||
| + | |||
| + | The second graphic shows S_cell VS O.D.. This plot allows the conparison of S_cell values between different cultures, that are supposed to reach the same level of growth not at the same time, but at the same O.D. value. | ||
| + | |||
| + | The third graphic shows the induction curve. The RPU value is calculated on S_cell values corresponding to O.D. values in exponential phase (typically, from 0.05 to 0.16). | ||
| + | The curve is obtained averaging in time S_cell values corresponding to exponential phase. | ||
| + | |||
| + | Error bars rapresent the minimum and maximum value of R.P.U. belonging to the range of O.D. in exponential phase. | ||
| + | |||
| + | |||
| + | ===='''Materials'''==== | ||
| + | *Long term glycerol stocks were stored at -80°C with a final glycerol concentration of 20% | ||
| + | *Antibiotics were: Ampicillin (Amp) 100 ug/ml. It was stored at -20°C in 1000x stocks. It was dissolved in water. | ||
| + | *LB medium was prepare with: 1% NaCl, 1% bactotryptone, 0.5% yeast extract. The medium was not buffered with NaOH. | ||
| + | *M9 supplemented medium was prepared according to: [http://openwetware.org/wiki/Knight:M9_supplemented_media Openwetware protocol]. | ||
| + | *aTc (Clontech) was dissolved in ethanol 50% and stored at -20°C in a 100 ug/ml stock. All the following dilutions were performed in water. | ||
===User Reviews=== | ===User Reviews=== | ||
Latest revision as of 13:37, 22 October 2009
Applications of BBa_K173005
BBa_K173005/BBa_Q04400 - tetR QPI - UNIPV-Pavia (Test performed by L. Pasotti, S. Zucca)
Description
This device takes PoPS as input and gives a PoPS output according to the transfer function of a logic inverter: tetR (BBa_C0040) is a repressor for tetR promoter (BBa_R0040), so high input signals give a low expression of tetR promoter and low input signals give a high expression of the promoter. We have characterized BBa_K173005 part (twin of BBa_Q04400) as a constitutive device, equivalent to tetR promoter, and as a genetic logic inverter. BBa_K173005 has been studied as constitutive device using BBa_K173008, which has been induced with different aTc concentrations in order to see if GFP levels changed as a function of aTc concentration. On the other hand, BBa_K173005 has been studied as a logic inverter by using BBa_K173008, BBa_K173009 and BBa_K173026, in which tetR expression is driven by constitutive promoters of different and measured strengths (no promoter, BBa_J23100 and BBa_J23118 respectively).
Characterization
Compatibility: E. coli TOP10 in pSB1A2
This part has been characterized only in M9 supplemented medium. In LB only a measure in absence of inducer has been performed.
| aTc concentration [ng/ml] |
LB | M9 supplemented | ||
| Doubling time [minutes] | RPU | Doubling time [minutes] | RPU | |
| 0 | 32 | 1.58 [ 1.52 ; 1.67] | 78 | 1.36 [ 1.22 ; 1.65] |
| 25 | not performed | not performed | 68 | 1.62 [ 1.49 ; 1.97] |
| 50 | not performed | not performed | 69 | 1.59 [ 1.36 ; 2.10] |
| 75 | not performed | not performed | 75 | 1.59 [ 1.32 ; 2.03] |
| 100 | not performed | not performed | 78 | 1.75 [ 1.57 ; 2.10] |
| 200 | not performed | not performed | 82 | 1.57 [ 1.35 ; 1.98] |
| 300 | not performed | not performed | 85 | 1.31 [ 1.21 ; 1.51] |
 BBa_K173008 Growth curves for BBa_K173008 in M9 |
 BBa_K173008(dGFP/dt)/O.D. in M9 |
 BBa_K173008 Induction curve of BBa_K173008 in M9 |
 BBa_K173008Logical inverter: RPU of BBa_K173008 in function of RPU of promoter used for tetR expression in LB |
The last image reports the inverter behaviour of tetR QPI component in LB medium. Considering RPU of promoter regulating tetR expression as input and RPU of tetR QPI device as output it is possible to characterize the logic inverter reported in figure. NB: RPU of promoters BBa_J23100 and BBa_J23118 have been calculated as reported in the BBa_J23100 Experience page, while the absence of promoter has been associated to RPU=0. When input (RPU of the promoter expressing tetR) is high, the output is low and the behavior is opposite for high input RPU.
Conclusions
The expected behaviour of this part was not to be affected by the presence of inducer, because the repressor should not be present. In fact, tetR production isn't regulated by any promoter and Ptet can work as a constitutive one.
Actually, the activity of promoter is not independent from aTc concentration, probably because of spurious transcription of tetR repressor in the high copy number plasmid. The experiments concerning this part have been performed in M9 supplemented medium. In LB medium the component has been tested only in absence of inducer and this has been useful to estimate three points of the transfer function of BBa_K173005 as a logic inverter.
We can say that the behaviour of BBa_K173005 as a logic inverter has been validated, even if more points of the transfer function have to be measured.
Growth conditions
Microplate reader experiments
- 8 ul of long term storage glycerol stock were inoculated in 5 ml of LB + suitable antibiotic in a 15 ml falcon tube and incubated at 37°C, 220 rpm for about 16 hours.
- The grown cultures were then diluted 1:100 in 5 ml of LB or M9 supplemented medium and incubated in the same conditions as before for about 4 hours.
- These new cultures were diluted to an O.D.600 of 0.02 (measured with a TECAN F200 microplate reader on a 200 ul of volume per well; it is not comparable with the 1 cm pathlength cuvette) in a sufficient amount of medium to fill all the desired microplate wells.
- These new dilutions were aliquoted in a flat-bottom 96-well microplate, avoiding to perform dynamic experiments in the microplate frame (see [http://2009.igem.org/Team:UNIPV-Pavia/Methods_Materials/Evaporation Frame effect section] for details). All the wells were filled with a 200 ul volume.
- If required, 2 ul of inducer were added to each single well.
- The microplate was incubated in the Tecan Infinite F200 microplate reader and fluorescence (when required) and absorbance were measured with this automatic protocol:
- 37°C constant for all the experiment;
- sampling time of 5 minutes;
- fluorescence gain of 50;
- O.D. filter was 600 nm;
- GFP filters were 485nm (ex) / 540nm (em);
- 15 seconds of linear shaking (3mm amplitude) followed by 10 seconds of waiting before the measurements in order to make a homogeneous culture.
- Variable experiment duration time (from 3 to 24 hours).
Data analysis
Growth curves
All our growth curves have been obtained subtracting for each time sample the broth O.D.600 measurement from that of the culture; broth was considered in the same conditions of the culture (e.g. induced with the same inducer concentration).
Doubling time
The natural logarithm of the growth curves (processed according to the above section) was computed and the linear phase (corresponding to the bacterial exponential growth phase) was isolated by visual inspection. Then the linear regression was performed in order to estimate the slope of the line m. Finally the doubling time was estimated as d=ln(2)/m [minutes].
In the case of multiple growth curves for a strain, the mean value of the processed curves was computed for each time sample before applying the above described procedure.
Relative Promoter Units (RPUs)
The RPUs are standard units proposed by Kelly J. et al., 2008, in which the transcriptional strength of a promoter can be measured using a reference standard, just like the ground in electric circuits.
RPUs have been computed as:
in which:
- phi is the considered promoter and J23101 is the reference standard promoter (taken from Anderson Promoter Collection);
- F is the blanked fluorescence of the culture, computed subtracting for each time sample fluorescence measure for negative control from that of culture, where the negative control is a non-fluorescent strain (in our experiment it is usually used TOP10 strain bearing BBa_B0032 or BBa_B0033, which are symmply RBSs do not have expression systems for reporter genes);
- ABS is the blanked absorbance (O.D.600) of the culture, computed as described in "Growth curves" section.
RPU measurement has the following advantages (under suitable conditions)
- it is proportional to PoPS (Polymerase Per Second), a very important parameter that expresses the transcription rate of a promoter;
- it uses a reference standard and so measurements can be compared between different laboratories.
The hypotheses on which RPU theory is based can be found in Kelly J. et al., 2008, as well as all the mathematical steps. From our point of view, the main hypotheses that have to be satisfied are the following:
- the reporter protein must have a half life higher than the experiment duration (we use GFPmut3, BBa_E0240, which has an estimated half life of at least 24 hours, and the experiments duration is always less than 7 hours);
- strain, plasmid copy number, antibiotic, growth medium, growth conditions, protein generator assembled downstream of the promoter must be the same in the promoter of interest and in J23101 reference standard.
- steady state must be valid, so (dF/dt)/ABS (proportional to the GFP synthesis rate per cell) must be constant.
Inducible systems
Every experiment is performed on the following cultures:
- the culture of interest (system studied expressing GFP)
- the benchmarck used to evaluate R.P.U. (BBa_K173001 measurement part, that is BBa_J23101 with BBa_E0240 downstream)
- a negative control (generally, BBa_B0033 RBS)
For inducible systems several plots are reported. The first plot is a panel containing 4 subplots, numerated this way:
| (1) | (2) |
| (3) |
Plot (1) contains growth curves of the cultures, after blank value has been removed. Every curve is calculated averaging on three replicates of the same culture and subtracting the blank for each time sample. Blank is calculated averaging the replicates of blank wells.
Plot (2) shows the logarithm of absorbance in exponential phase of bacterial growth, determined by a visual inspection of log-plots. These values are used to evaluate doubling time and R.P.U..
Plot (3) contains (dGFP/dt)/O.D., the value named S_cell in Kelly J. et al., 2008 procedure for RPU evaluation.
In these plots are reported black veritcal lines that define the range of values used to evaluate RPU. It is important to underline, as explained in next paragraph, that RPU are calculated on cultures at the same O.D. level, not at the same time.
The second graphic shows S_cell VS O.D.. This plot allows the conparison of S_cell values between different cultures, that are supposed to reach the same level of growth not at the same time, but at the same O.D. value.
The third graphic shows the induction curve. The RPU value is calculated on S_cell values corresponding to O.D. values in exponential phase (typically, from 0.05 to 0.16). The curve is obtained averaging in time S_cell values corresponding to exponential phase.
Error bars rapresent the minimum and maximum value of R.P.U. belonging to the range of O.D. in exponential phase.
Materials
- Long term glycerol stocks were stored at -80°C with a final glycerol concentration of 20%
- Antibiotics were: Ampicillin (Amp) 100 ug/ml. It was stored at -20°C in 1000x stocks. It was dissolved in water.
- LB medium was prepare with: 1% NaCl, 1% bactotryptone, 0.5% yeast extract. The medium was not buffered with NaOH.
- M9 supplemented medium was prepared according to: [http://openwetware.org/wiki/Knight:M9_supplemented_media Openwetware protocol].
- aTc (Clontech) was dissolved in ethanol 50% and stored at -20°C in a 100 ug/ml stock. All the following dilutions were performed in water.
User Reviews
UNIQ84612c31852e7771-partinfo-0000001B-QINU UNIQ84612c31852e7771-partinfo-0000001C-QINU