Difference between revisions of "Part:BBa K1761000"
(change picture size) |
(→DBCO-PEG4-TAMRA Confirmation) |
||
| (8 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K1761000 short</partinfo> | <partinfo>BBa_K1761000 short</partinfo> | ||
| − | OmpX is an outer membrane protein with the C- and N-termini in the intracellulair domain. To be able to use OmpX as a scaffold, a | + | OmpX is an outer membrane protein with the C- and N-termini in the intracellulair domain. To be able to use OmpX as a scaffold, a unnatural amino acid needs to be introduced. This can be done by implementing the amber stop codon TAG in one of the loops of OmpX via a mutation. With a specific tRNA an azide-functionalized amino acid can be built in, which can be used for the SPAAC click chemistry reaction with DBCO functionalized groups. |
| Line 13: | Line 13: | ||
== Gene Design == | == Gene Design == | ||
| − | OmpX was identified and characterized by Mecsas et al and is 513 bp long. It contains a putative signal sequence, which makes sure that OmpX will be in the membrane. [2] To be able to use OmpX as a scaffold, one needs to integrate the | + | OmpX was identified and characterized by Mecsas et al. and is 513 bp long. It contains a putative signal sequence, which makes sure that OmpX will be in the membrane. [2] To be able to use OmpX as a scaffold, one needs to integrate the unnatural amino acid. To do so, some modifications must be made to this part. Within the original OmpX part, a codon was mutated into the amber stop codon TAG. This codon will be incorporated in the protruding loops of OmpX, loop 2 and loop 3 (see Figure 2). These loops were chosen because they are easily accessible and are not part of the beta-barrel of OmpX [3]. We believe that mutations in these loops will not disturb the secondary structure of OmpX. With a specific tRNA it is possible to implement a unnatural amino acid at the place of the TAG codon. For more information on which specific mutations we made, see our Project Design page [http://2015.igem.org/Team:TU_Eindhoven/Project/Design]. |
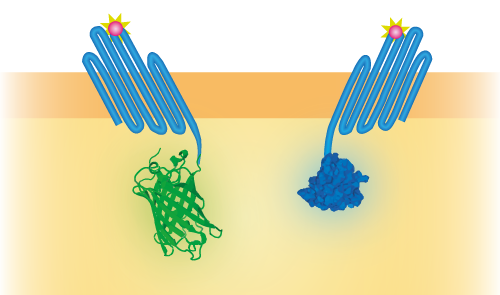
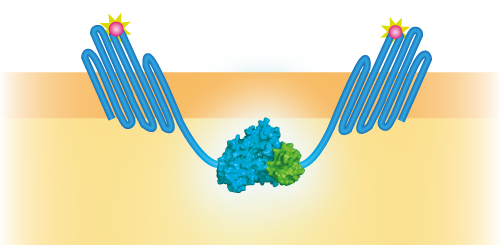
| − | To be able to use OmpX in a sensor system, we attached several intracellular domains, namely NanoLuc [https://parts.igem.org/Part:BBa_K1761002], mNeonGreen [https://parts.igem.org/Part:BBa_K1761001]and | + | To be able to use OmpX in a sensor system, we attached several intracellular domains, namely NanoLuc [https://parts.igem.org/Part:BBa_K1761002], mNeonGreen [https://parts.igem.org/Part:BBa_K1761001], LargeBit [https://parts.igem.org/Part:BBa_K1761005] and SmallBit [https://parts.igem.org/Part:BBa_K1761006]. These domains can interact with each other in different ways. NanoLuc and mNeonGreen form a BRET pair (see Figure 2) and LargeBit and SmallBit form NanoBit, a luciferase (see Figure 3). These constructs were attached to OmpX with a linker and were inserted in a pETDuet-1 vector that has two multiple cloning sites (MCS-1 and MCS-2). |
| + | [[File:TU_Eindhoven_Constructs_OmpX_NG_and_OmpX_NL.png]] | ||
| + | ''Figure 2: Schematical overview of the expressed OmpX with mNeonGreen and OmpX with NanoLuc in the outer membrane of E.coli. The mNeonGreen and NanoLuc domains form a BRET pair.'' | ||
| + | |||
| + | [[File:TU_Eindhoven_OmpX_LgBiT_and_OmpX_SmBiT.png]] | ||
| + | |||
| + | ''Figure 3: Schematical overview of the NanoBit construct.'' | ||
== Sequence == | == Sequence == | ||
| − | The sequence of our OmpX part has been verified by sequencing at StarSeq. It contains the prefix and suffix with the correct restriction sites (EcoRI, XbaI, SpeI and PstI). OmpX itself is 513 bp long. | + | The sequence of our OmpX part (see Figure 4) has been verified by sequencing at StarSeq. It contains the prefix and suffix with the correct restriction sites (EcoRI, XbaI, SpeI and PstI). OmpX itself is 513 bp long. |
| + | [[File:TU Eindhoven OmpX SnapGene.png|800px]] | ||
| + | |||
| + | ''Figure 4: Snapgene plasmid overview of the BioBrick part BBa_K1761000. It shows the pSB1C3 vector with the prefix (containing the restriction sites EcoRI and XbaI), wildtype OmpX and the suffix (containting the restiction sites SpeI and PstI). '' | ||
| + | |||
<!-- --> | <!-- --> | ||
| Line 28: | Line 38: | ||
== Characterization == | == Characterization == | ||
| − | When mutated with the amber stop codon TAG, | + | When mutated with the amber stop codon TAG, an unnatural amino acid with an azide-functionalized group can be expressed. After the expression of this amino acid, OmpX can covalently bind almost anything, as long as it contains a DBCO-functionalized group. The binding finds place by using a bio-orthogonal “click-reaction" (SPAAC chemistry). To test the functionality of this “click-reaction", some experiments were done by clicking DBCO-PEG4-TAMRA at the surface. |
| − | For all the experiments we used the following vectors: pETDuet-1 with one or two construct(s) inserted (OmpX + intracellular protein) and pEVOL-pAzF (tRNA + tRNA synthetase). Both vectors were transformed into BL21(DE3). The expression was introduced by adding arabinose, IPTG and the | + | For all the experiments we used the following vectors: pETDuet-1 with one or two construct(s) inserted (OmpX + intracellular protein) and pEVOL-pAzF (tRNA + tRNA synthetase, see BBa_K1492002 [https://parts.igem.org/Part:BBa_K1492002]). Both vectors were transformed into BL21(DE3). The expression was introduced by adding arabinose, IPTG and the unnatural amino acid. |
| − | + | ||
== DBCO-PEG4-TAMRA Confirmation == | == DBCO-PEG4-TAMRA Confirmation == | ||
| − | To confirm whether OmpX is in the membrane and whether or not the | + | To confirm whether OmpX is in the membrane and whether or not the unnatural amino acid is being incorporated into OmpX, DBCO-PEG4-TAMRA was used. TAMRA is a fluorescent dye that can be used to verify the “click-reaction". If the unnatural amino acid is present, DBCO-PEG4-TAMRA should “click” to the transmembrane protein OmpX and stay there. This can be analyzed with FACS. For more information about how to perform FACS experiments, see our Protocol Page [http://2015.igem.org/Team:TU_Eindhoven/Project/Protocols]. |
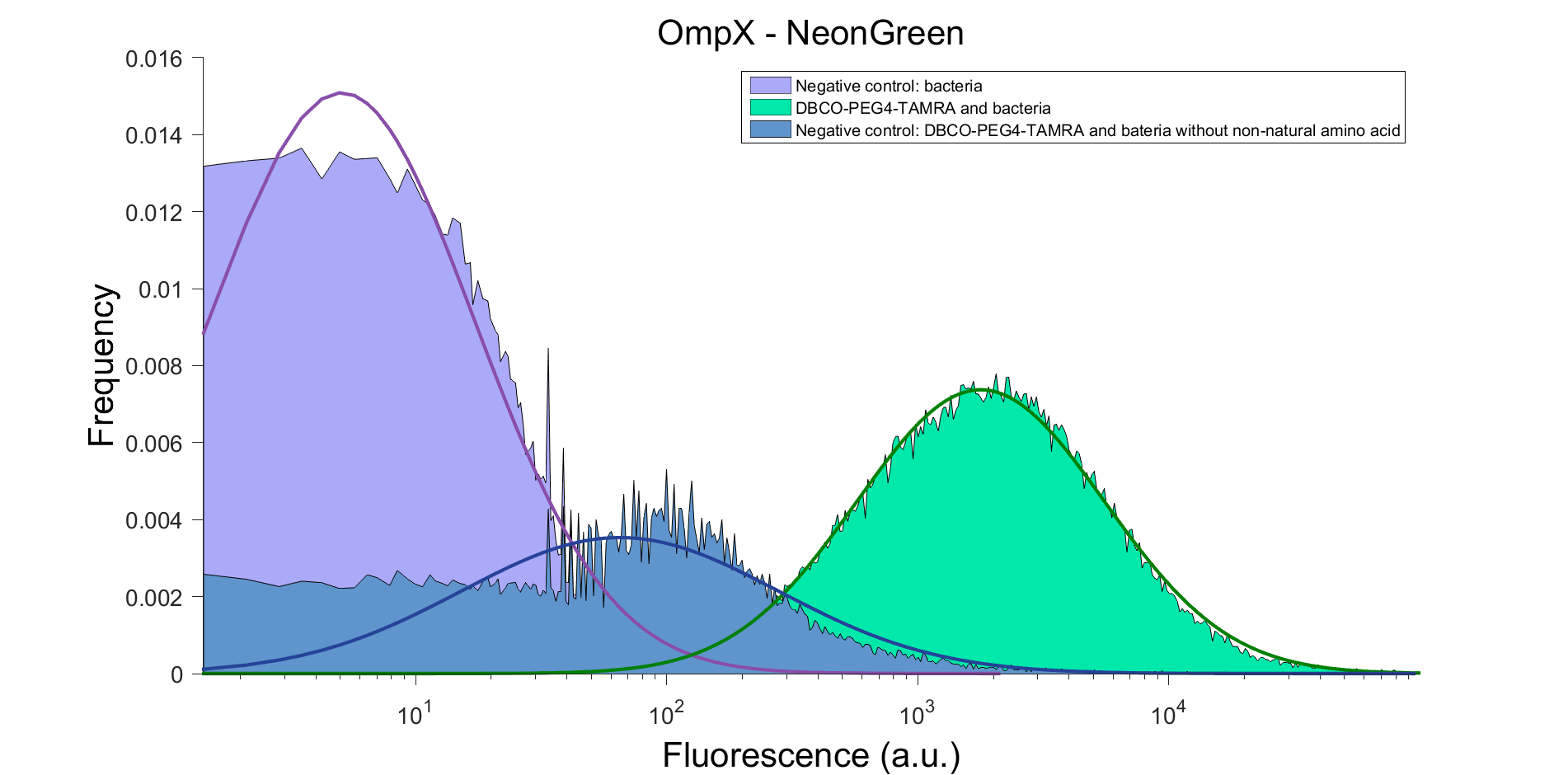
| − | To verify that OmpX is in the membrane, we used the OmpX – NanoLuc and OmpX – mNeonGreen constructs. These gave the following results after clicking with DBCO-PEG4-TAMRA (see Figure | + | To verify that OmpX is in the membrane, we used the OmpX – NanoLuc and OmpX – mNeonGreen constructs. These gave the following results after clicking with DBCO-PEG4-TAMRA (see Figure 5 and 6). From this it can be concluded that OmpX is in the membrane and that the “click” reaction works. |
[[File:TU Eindhoven FACS result OmpX NL.png|800px]] | [[File:TU Eindhoven FACS result OmpX NL.png|800px]] | ||
| − | ''Figure | + | ''Figure 5: FACS result of OmpX – NanoLuc.'' |
[[File:TU Eindhoven FACS result OmpX NG.png|800px]] | [[File:TU Eindhoven FACS result OmpX NG.png|800px]] | ||
| − | ''Figure | + | ''Figure 6: FACS result of OmpX - mNeonGreen'' |
| + | |||
| + | == Scaffold for signaling at the membrane == | ||
| + | |||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
| Line 48: | Line 60: | ||
<partinfo>BBa_K1761000 parameters</partinfo> | <partinfo>BBa_K1761000 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| − | == | + | |
| + | == References == | ||
[1] J. Vogt and G. E. Schulz, “The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence.,” Structure, vol. 7, no. 10, pp. 1301–9, Oct. 1999. | [1] J. Vogt and G. E. Schulz, “The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence.,” Structure, vol. 7, no. 10, pp. 1301–9, Oct. 1999. | ||
Latest revision as of 01:48, 19 September 2015
Outer Membrane Protein X (OmpX)
OmpX is an outer membrane protein with the C- and N-termini in the intracellulair domain. To be able to use OmpX as a scaffold, a unnatural amino acid needs to be introduced. This can be done by implementing the amber stop codon TAG in one of the loops of OmpX via a mutation. With a specific tRNA an azide-functionalized amino acid can be built in, which can be used for the SPAAC click chemistry reaction with DBCO functionalized groups.
Usage and Biology
OmpX, or Outer Membrane Protein X, belongs to a family of highly conserved bacterial proteins that promote bacterial adhesion to and entry into mammalian cells. It presents both C- and N-termini in the intracellular domain (see Figure 1). OmpX consists of an eight-stranded antiparallel all-next-neighbor β barrel. The core of the protein consists of an extended hydrogen-bonding network of highly conserved residues (see Figure 1). [1]
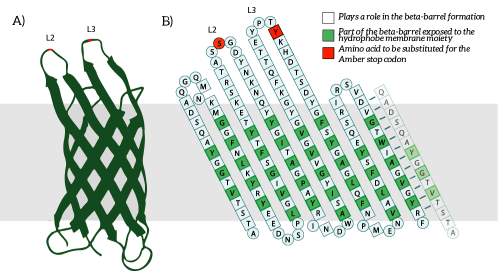
Figure 1: A) The OmpX protein structure has been elucidated through NMR and X-ray crystallography, B) The square residues are important for the secondary structure of OmpX. To keep the structure intact, we introduce an amber stop codon in one of the protruding loops. Figure 5B is adapted from [1].
Gene Design
OmpX was identified and characterized by Mecsas et al. and is 513 bp long. It contains a putative signal sequence, which makes sure that OmpX will be in the membrane. [2] To be able to use OmpX as a scaffold, one needs to integrate the unnatural amino acid. To do so, some modifications must be made to this part. Within the original OmpX part, a codon was mutated into the amber stop codon TAG. This codon will be incorporated in the protruding loops of OmpX, loop 2 and loop 3 (see Figure 2). These loops were chosen because they are easily accessible and are not part of the beta-barrel of OmpX [3]. We believe that mutations in these loops will not disturb the secondary structure of OmpX. With a specific tRNA it is possible to implement a unnatural amino acid at the place of the TAG codon. For more information on which specific mutations we made, see our Project Design page [http://2015.igem.org/Team:TU_Eindhoven/Project/Design].
To be able to use OmpX in a sensor system, we attached several intracellular domains, namely NanoLuc [1], mNeonGreen [2], LargeBit [3] and SmallBit [4]. These domains can interact with each other in different ways. NanoLuc and mNeonGreen form a BRET pair (see Figure 2) and LargeBit and SmallBit form NanoBit, a luciferase (see Figure 3). These constructs were attached to OmpX with a linker and were inserted in a pETDuet-1 vector that has two multiple cloning sites (MCS-1 and MCS-2).
Figure 2: Schematical overview of the expressed OmpX with mNeonGreen and OmpX with NanoLuc in the outer membrane of E.coli. The mNeonGreen and NanoLuc domains form a BRET pair.
Figure 3: Schematical overview of the NanoBit construct.
Sequence
The sequence of our OmpX part (see Figure 4) has been verified by sequencing at StarSeq. It contains the prefix and suffix with the correct restriction sites (EcoRI, XbaI, SpeI and PstI). OmpX itself is 513 bp long.
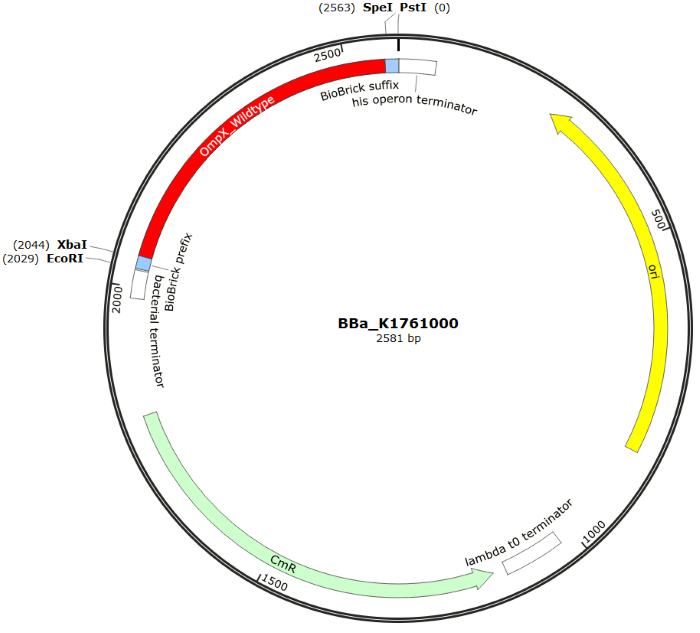
Figure 4: Snapgene plasmid overview of the BioBrick part BBa_K1761000. It shows the pSB1C3 vector with the prefix (containing the restriction sites EcoRI and XbaI), wildtype OmpX and the suffix (containting the restiction sites SpeI and PstI).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterization
When mutated with the amber stop codon TAG, an unnatural amino acid with an azide-functionalized group can be expressed. After the expression of this amino acid, OmpX can covalently bind almost anything, as long as it contains a DBCO-functionalized group. The binding finds place by using a bio-orthogonal “click-reaction" (SPAAC chemistry). To test the functionality of this “click-reaction", some experiments were done by clicking DBCO-PEG4-TAMRA at the surface. For all the experiments we used the following vectors: pETDuet-1 with one or two construct(s) inserted (OmpX + intracellular protein) and pEVOL-pAzF (tRNA + tRNA synthetase, see BBa_K1492002 [5]). Both vectors were transformed into BL21(DE3). The expression was introduced by adding arabinose, IPTG and the unnatural amino acid.
DBCO-PEG4-TAMRA Confirmation
To confirm whether OmpX is in the membrane and whether or not the unnatural amino acid is being incorporated into OmpX, DBCO-PEG4-TAMRA was used. TAMRA is a fluorescent dye that can be used to verify the “click-reaction". If the unnatural amino acid is present, DBCO-PEG4-TAMRA should “click” to the transmembrane protein OmpX and stay there. This can be analyzed with FACS. For more information about how to perform FACS experiments, see our Protocol Page [http://2015.igem.org/Team:TU_Eindhoven/Project/Protocols].
To verify that OmpX is in the membrane, we used the OmpX – NanoLuc and OmpX – mNeonGreen constructs. These gave the following results after clicking with DBCO-PEG4-TAMRA (see Figure 5 and 6). From this it can be concluded that OmpX is in the membrane and that the “click” reaction works.
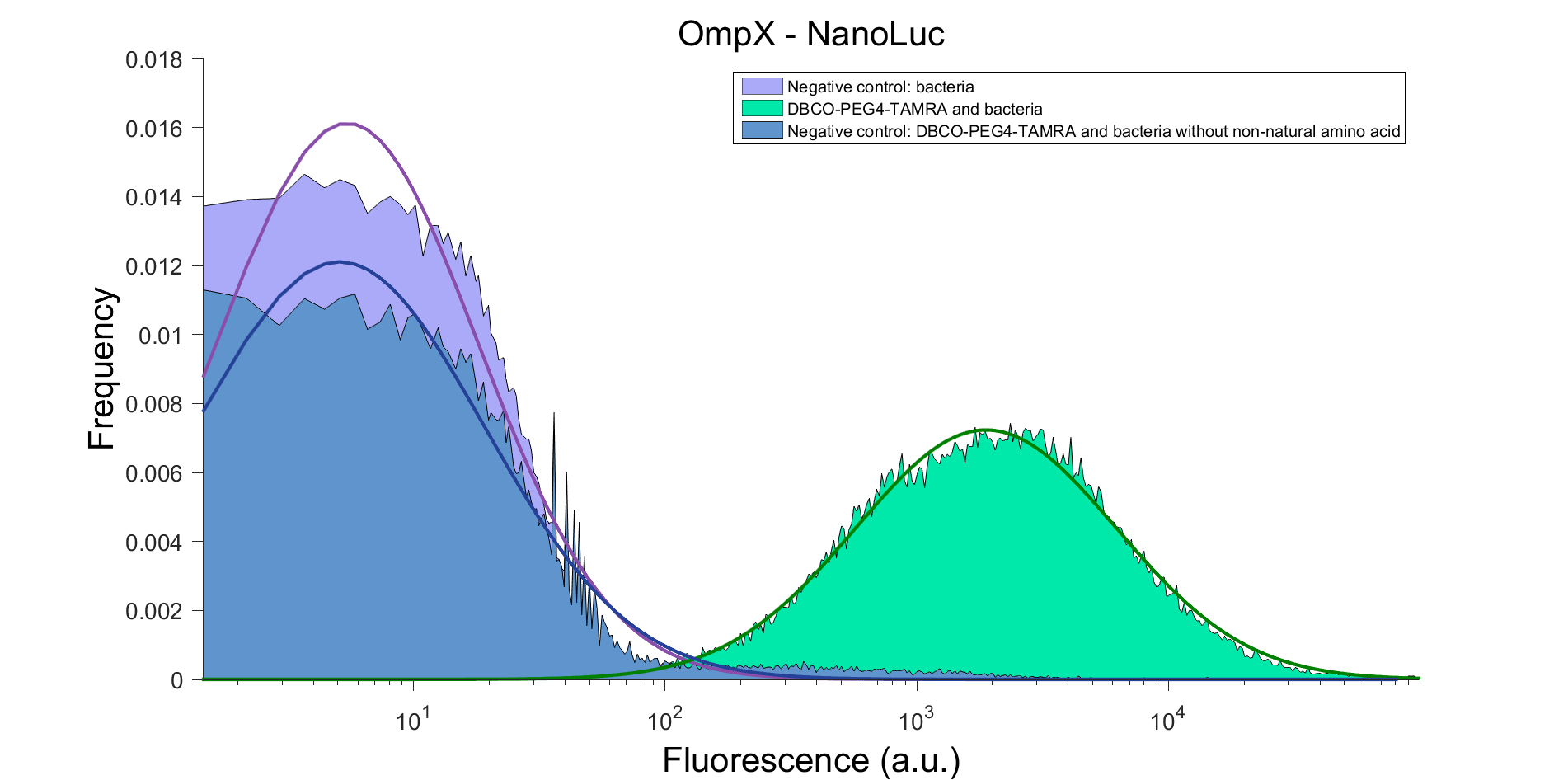
Figure 5: FACS result of OmpX – NanoLuc.
Figure 6: FACS result of OmpX - mNeonGreen
Scaffold for signaling at the membrane
References
[1] J. Vogt and G. E. Schulz, “The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence.,” Structure, vol. 7, no. 10, pp. 1301–9, Oct. 1999.
[2] J. Mescas, R. Welch, J.W. Erickson and C.A. Gross, “Identification and characterization of an Outer Membrane Protein, OmpX, in Escherichia coli that is homologous to a family of outer membrane proteins including ail of Yersinia enterocolitica.,” Journal of Bacteriology, vol. 177, no. 3, pp. 799-804, Nov. 1994.
[3] J. J. Rice, A. Schohn, P. H. Bessette, K. T. Boulware, and P. S. Daugherty, “Bacterial display using circularly permuted outer membrane protein OmpX yields high affinity peptide ligands.,” Protein Sci., vol. 15, no. 4, pp. 825–36, Apr. 2006.