Difference between revisions of "Part:BBa K909007"
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
| − | <partinfo>BBa_K909007 short</partinfo> | + | <partinfo>BBa_K909007 short</partinfo> |
| − | |||
| − | |||
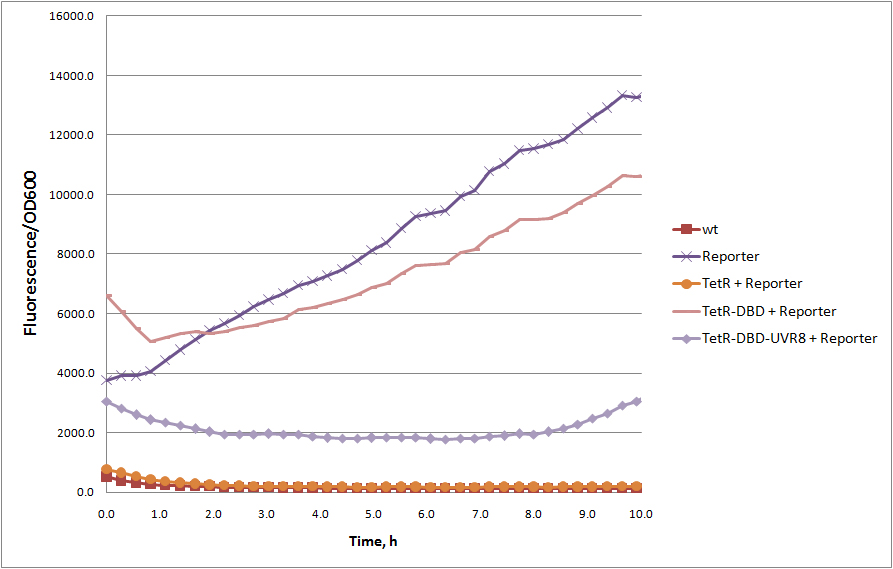
| − | <!-- Add more about the biology of this part here | + | Truncated version of tetracycline repressor TetR, consisting of 1 – 127 amino acids, containing DNA binding domain. The rest of the protein, responsible for tetR dimerization, were removed. Without dimerization domain, tetR-DBD fails to bind TetR responsive promoter efficiently and unable to repress transcription of reporter protein (see figure). However, TetR-DBD fusion with dimerazing proteins, e.g. UVR8 protein (<partinfo>BBa_K909008</partinfo>), restores TetR-DBD promoter binding. |
| + | |||
| + | [[Image:TetR, tetR-DBD, tetR-DBD-UVR8 represion.jpg|frameless|upright=2.5|center|thumb|GFP repression (reporter) by full length TetR, TetR-DBD and dimerizing TetR-DBD-UVR8 fusion | ||
| + | ]] | ||
| + | |||
| + | <!-- Add more about the biology of this part here --> | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | |||
| + | We introduced BamHI site at C terminus of tetR-DBD for an easy in frame fusions with other proteins. This can easily be used as a two hybrid system to detect homo/heterodimers in ''E. coli'', and, in principle, one can use these fusions to turn protein-protein interaction into the repression of transcription. Furthermore, inducible dimerization can lead to a novel switch like behaviors of the system (e.g. [http://2012.igem.org/Team:ETH_Zurich/UVR8 ETH Zurich 2012 team] created a novel UV-B inducible-ON switch). | ||
| + | |||
| + | |||
<!-- --> | <!-- --> | ||
| Line 14: | Line 20: | ||
| − | + | ||
| − | + | ||
| + | ==Functional Parameters: Austin_UTexas== | ||
| + | <html> | ||
| + | <body> | ||
<partinfo>BBa_K909007 parameters</partinfo> | <partinfo>BBa_K909007 parameters</partinfo> | ||
| − | < | + | <h3><center>Burden Imposed by this Part:</center></h3> |
| + | <figure> | ||
| + | <div class = "center"> | ||
| + | <center><img src = "https://static.igem.org/mediawiki/parts/f/fa/T--Austin_Utexas--no_burden_icon.png" style = "width:160px;height:120px"></center> | ||
| + | </div> | ||
| + | <figcaption><center><b>Burden Value: -2.9 ± 1.8% </b></center></figcaption> | ||
| + | </figure> | ||
| + | <p> Burden is the percent reduction in the growth rate of <i>E. coli</i> cells transformed with a plasmid containing this BioBrick (± values are 95% confidence limits). This BioBrick did not exhibit a burden that was significantly greater than zero (i.e., it appears to have little to no impact on growth). Therefore, users can depend on this part to remain stable for many bacterial cell divisions and in large culture volumes. Refer to any one of the | ||
| + | <a href="https://parts.igem.org/Part:BBa_K3174002">BBa_K3174002</a> - <a href="https://parts.igem.org/Part:BBa_K3174007">BBa_K3174007</a> pages for more information on the methods, an explanation of the sources of burden, and other conclusions from a large-scale measurement project conducted by the <a href="http://2019.igem.org/Team:Austin_UTexas">2019 Austin_UTexas team</a>.</p> | ||
| + | <p>This functional parameter was added by the <a href="https://2020.igem.org/Team:Austin_UTexas/Contribution">2020 Austin_UTexas team.</a></p> | ||
| + | </body> | ||
| + | </html> | ||
Latest revision as of 02:51, 26 August 2020
TetR DNA binding domain containing BamHI site for protein fusions
Truncated version of tetracycline repressor TetR, consisting of 1 – 127 amino acids, containing DNA binding domain. The rest of the protein, responsible for tetR dimerization, were removed. Without dimerization domain, tetR-DBD fails to bind TetR responsive promoter efficiently and unable to repress transcription of reporter protein (see figure). However, TetR-DBD fusion with dimerazing proteins, e.g. UVR8 protein (BBa_K909008), restores TetR-DBD promoter binding.
Usage and Biology
We introduced BamHI site at C terminus of tetR-DBD for an easy in frame fusions with other proteins. This can easily be used as a two hybrid system to detect homo/heterodimers in E. coli, and, in principle, one can use these fusions to turn protein-protein interaction into the repression of transcription. Furthermore, inducible dimerization can lead to a novel switch like behaviors of the system (e.g. [http://2012.igem.org/Team:ETH_Zurich/UVR8 ETH Zurich 2012 team] created a novel UV-B inducible-ON switch).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 382
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Functional Parameters: Austin_UTexas
Burden Imposed by this Part:

Burden is the percent reduction in the growth rate of E. coli cells transformed with a plasmid containing this BioBrick (± values are 95% confidence limits). This BioBrick did not exhibit a burden that was significantly greater than zero (i.e., it appears to have little to no impact on growth). Therefore, users can depend on this part to remain stable for many bacterial cell divisions and in large culture volumes. Refer to any one of the BBa_K3174002 - BBa_K3174007 pages for more information on the methods, an explanation of the sources of burden, and other conclusions from a large-scale measurement project conducted by the 2019 Austin_UTexas team.
This functional parameter was added by the 2020 Austin_UTexas team.