Difference between revisions of "Part:BBa I757010:Experience"
UP Sebastian (Talk | contribs) (→User Reviews) |
Akatsaouni (Talk | contribs) (→Thessaly 2019 Characterization) |
||
| (12 intermediate revisions by 4 users not shown) | |||
| Line 4: | Line 4: | ||
===Applications of BBa_I757010=== | ===Applications of BBa_I757010=== | ||
| + | Done by iGEM11 Potsdam_Bioware<br> | ||
| + | |||
This beta lactamase was used in combination with the TorA signal sequence (BBa_K208005) as a new protease activity detector device. The BioBrick parts BBa_K627012 and BBa_K627013 consist of the TorA signal sequence followed by a short peptide sequence used as cleavage site for different proteases (TEV (BBa_K627008-BBa_K627010) and 14_3C (BBa_K627011)) and ends with the beta lactamase.<br> | This beta lactamase was used in combination with the TorA signal sequence (BBa_K208005) as a new protease activity detector device. The BioBrick parts BBa_K627012 and BBa_K627013 consist of the TorA signal sequence followed by a short peptide sequence used as cleavage site for different proteases (TEV (BBa_K627008-BBa_K627010) and 14_3C (BBa_K627011)) and ends with the beta lactamase.<br> | ||
| − | When <i>E.coli XL1 blue</i> where transformed with our construct, the cells were able to survive at ampicillin concentrations up to 400 µg/ml, when incubated on agar plates over night at 37°C | + | When <i>E.coli XL1 blue</i> where transformed with our construct, the cells were able to survive at ampicillin concentrations up to 400 µg/ml, when incubated on agar plates over night at 37°C or at 30 °C.<br> |
| + | |||
| + | ===Thessaly 2019 Characterization=== | ||
| + | <p id="10">Thessaly 2019 sought to <b>characterize</b> the coding sequence of <b>TEM-optimized β-lactamase</b> (<partinfo>BBa_I757010</partinfo>) <b>under the regulation of the constituve Anderson Family promoters</b> <partinfo>BBa_J23100</partinfo>, <partinfo>BBa_J23105</partinfo>, <partinfo>BBa_J23106</partinfo>, <partinfo>BBa_J23119</partinfo>. β-lactamase is an enzyme that hydrolyses β-lactams (e.g. ampicillin) and is naturally found in prokaryotic cells. A colorimetric assay has been developed using nitrocefin as a substrate which after hydrolysis from β-lactamase changes the reaction color, from yellow (380nm) to red (490nm).</p> | ||
| + | |||
| + | To achieve that, the coding sequence was assembled with each promoter, a <b>universal RBS</b> (<partinfo>BBa_B0034</partinfo>) and a <b>double terminator</b>(<partinfo>BBa_B0015</partinfo>). The parts were cloned in pSB1C3 and pSB1K3 and transformed into <i>E. coli DH5a</i> competent cells. | ||
| + | |||
| + | In the photo below you can see the results of the primer addition using <b>overhang PCR</b>: | ||
| + | |||
| + | <html> | ||
| + | <head> | ||
| + | <title>HTML img Tag</title> | ||
| + | </head> | ||
| + | |||
| + | <body> | ||
| + | <img src="https://2019.igem.org/wiki/images/3/34/T--Thessaly--Contribution_gel.png" class= "center" width="300" | ||
| + | height="300"> | ||
| + | <p style="text-align: justify; font-size: 14px; font-family: MuliLight; color: black; margin-left: auto; margin-right: auto;"><b>Figure 1.</b> The results obtained after the PCR with the overhang primers for the different promoters of the Anderson family. We tested different annealing temperatures (45, 47 & 53℃) aiming for clear results. The expected band is at 1119bp and the ladder used was the 100bp DNA ladder by NEB.</p> | ||
| + | </body> | ||
| + | </html> | ||
| + | |||
| + | |||
| + | <p>For protein expression, the plasmids were transformed into <i>E. coli BL21 (DE3)</i> competent cells.</p> | ||
| + | |||
| + | |||
| + | For the β-lactamase assay, we set up the following experimental design: | ||
| + | |||
| + | 1. Grow BL21 (DE3) pre-culture overnight in 5ml LB (~16h) at a shaking incubator, 37℃ / 210rpm | ||
| + | |||
| + | 2. The following morning, measure the OD600 of overnight cultures | ||
| + | |||
| + | 3. Dilute all cultures to OD600¬ = 0.05 in M9 minimal medium | ||
| + | |||
| + | 4. Grow cells 37℃ /210 RPM until OD600=0.4-0.6 (~2h) | ||
| + | |||
| + | 5. Dilute all cells to the same OD600 (e.g. 0.4) | ||
| + | |||
| + | 6. Load 160 of culture in a 96-well plate (do triplicates). Add 40 ul 0.5 uM nitrocefin for a final concentration of 100nM | ||
| + | |||
| + | 7. Measure the absorbance at 490nm (for nitrocefin hydrolysis) and 600nm (for cell growth) every 30 seconds for 2 hours in a microplate reader. Shake between readings. Because plateau was reached within the first 30 minutes of the reaction, only those are depicted in the graph. | ||
| + | |||
| + | To ensure that the absorbance shown corresponds only to enzymatic activity by β-lactamase, <b>we included 3 controls in the experiment</b>. | ||
| + | The first control has <b>M9 medium only</b> (no cells) and nitrocefin, the second has <b>empty BL21 (DE3) cells (no plasmid)</b> and nitrocefin, while the third has <b>BL21 (DE3) cells containing the plasmid but not the part (empty plasmid)</b>. | ||
| + | To obtain comparable results, we normalized all values by dividing OD490 by OD600. | ||
| + | |||
| + | |||
| + | The results are shown in the graph below | ||
| + | |||
| + | <html> | ||
| + | <head> | ||
| + | <title>HTML img Tag</title> | ||
| + | </head> | ||
| + | |||
| + | <body> | ||
| + | <img src="https://static.igem.org/mediawiki/parts/6/65/T--Thessaly--Graph-contrib.png" class= "center" width="800" | ||
| + | height="508"> | ||
| + | <p style="text-align: justify; font-size: 14px; font-family: MuliLight; color: black; margin-left: auto; margin-right: auto;"><b>Figure 2.</b> The hydrolysis of nitrocefin enabled by the expression of the β-lactamase gene, under the control of different promoters (J23100, J23105, J23106 & J23119) of the Anderson family. The substrate (nitrocefin) hydrolysis (490nm) is divided by cell growth (600nm), in order to normalize all values.</p> | ||
| + | </body> | ||
| + | </html> | ||
| + | |||
| + | <p> The maximum expression of β-lactamase was observed under control of the J23119 (brown line) which is the wild type promoter of the Anderson family. The expression is reduced with the J23100 and J23106 (yellow and purple line respectively), while the lowest expression levels were observed with the J23105 promoter (blue line). These results are in accordance with those from previous teams that measured fluorescence and the same pattern is observed. The controls conditions (pSB1C3 and BL21, or light purple and light blue respectively) confirm that the absorbance measured derives from β-lactamase activity only, both quantitatively and visually.</p> | ||
| + | |||
| + | Below you can see the 96-well plate of the assay: | ||
| + | |||
| + | <html> | ||
| + | <head> | ||
| + | <title>HTML img Tag</title> | ||
| + | </head> | ||
| + | |||
| + | <body> | ||
| + | <img src="https://static.igem.org/mediawiki/parts/7/7b/T--Thessaly--plate_reader_contribution.png" class= "center" width="800" | ||
| + | height="467"> | ||
| + | <p style="text-align: justify; font-size: 14px; font-family: MuliLight; color: black; margin-left: auto; margin-right: auto;"><b>Figure 3.</b>The observed color change due to the hydrolyzation of nitrocefin due to the production of β-lactamase, after a 2-hour enzymatic assay.</p> | ||
| + | </body> | ||
| + | </html> | ||
| + | |||
| + | <p><b>Note that the picture was taken after the plate-reader assay was completed and all conditions had reached a plateau, except the controls.</b></p> | ||
===User Reviews=== | ===User Reviews=== | ||
| Line 20: | Line 98: | ||
<!-- End of the user review template --> | <!-- End of the user review template --> | ||
<!-- DON'T DELETE --><partinfo>BBa_I757010 EndReviews</partinfo> | <!-- DON'T DELETE --><partinfo>BBa_I757010 EndReviews</partinfo> | ||
| − | The lactamases shows great activity at 37°C and 30°C incubation over night on agar plates. The transformed <i>E.coli</i> cells were able to survive with ampicillin concentrations up to 400 µg/ml at 37°C and | + | The lactamases shows great activity at 37°C and 30°C incubation over night on agar plates. The transformed <i>E.coli</i> cells were able to survive with ampicillin concentrations up to 400 µg/ml at 37°C and at 30°C. |
Latest revision as of 14:01, 21 October 2019
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_I757010
Done by iGEM11 Potsdam_Bioware
This beta lactamase was used in combination with the TorA signal sequence (BBa_K208005) as a new protease activity detector device. The BioBrick parts BBa_K627012 and BBa_K627013 consist of the TorA signal sequence followed by a short peptide sequence used as cleavage site for different proteases (TEV (BBa_K627008-BBa_K627010) and 14_3C (BBa_K627011)) and ends with the beta lactamase.
When E.coli XL1 blue where transformed with our construct, the cells were able to survive at ampicillin concentrations up to 400 µg/ml, when incubated on agar plates over night at 37°C or at 30 °C.
Thessaly 2019 Characterization
Thessaly 2019 sought to characterize the coding sequence of TEM-optimized β-lactamase (BBa_I757010) under the regulation of the constituve Anderson Family promoters BBa_J23100, BBa_J23105, BBa_J23106, BBa_J23119. β-lactamase is an enzyme that hydrolyses β-lactams (e.g. ampicillin) and is naturally found in prokaryotic cells. A colorimetric assay has been developed using nitrocefin as a substrate which after hydrolysis from β-lactamase changes the reaction color, from yellow (380nm) to red (490nm).
To achieve that, the coding sequence was assembled with each promoter, a universal RBS (BBa_B0034) and a double terminator(BBa_B0015). The parts were cloned in pSB1C3 and pSB1K3 and transformed into E. coli DH5a competent cells.
In the photo below you can see the results of the primer addition using overhang PCR:
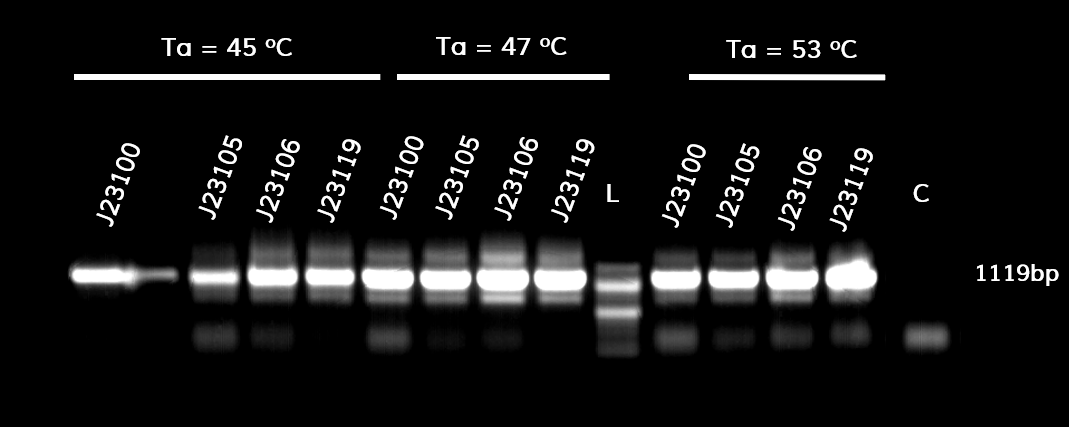
Figure 1. The results obtained after the PCR with the overhang primers for the different promoters of the Anderson family. We tested different annealing temperatures (45, 47 & 53℃) aiming for clear results. The expected band is at 1119bp and the ladder used was the 100bp DNA ladder by NEB.
For protein expression, the plasmids were transformed into E. coli BL21 (DE3) competent cells.
For the β-lactamase assay, we set up the following experimental design:
1. Grow BL21 (DE3) pre-culture overnight in 5ml LB (~16h) at a shaking incubator, 37℃ / 210rpm
2. The following morning, measure the OD600 of overnight cultures
3. Dilute all cultures to OD600¬ = 0.05 in M9 minimal medium
4. Grow cells 37℃ /210 RPM until OD600=0.4-0.6 (~2h)
5. Dilute all cells to the same OD600 (e.g. 0.4)
6. Load 160 of culture in a 96-well plate (do triplicates). Add 40 ul 0.5 uM nitrocefin for a final concentration of 100nM
7. Measure the absorbance at 490nm (for nitrocefin hydrolysis) and 600nm (for cell growth) every 30 seconds for 2 hours in a microplate reader. Shake between readings. Because plateau was reached within the first 30 minutes of the reaction, only those are depicted in the graph.
To ensure that the absorbance shown corresponds only to enzymatic activity by β-lactamase, we included 3 controls in the experiment. The first control has M9 medium only (no cells) and nitrocefin, the second has empty BL21 (DE3) cells (no plasmid) and nitrocefin, while the third has BL21 (DE3) cells containing the plasmid but not the part (empty plasmid). To obtain comparable results, we normalized all values by dividing OD490 by OD600.
The results are shown in the graph below

Figure 2. The hydrolysis of nitrocefin enabled by the expression of the β-lactamase gene, under the control of different promoters (J23100, J23105, J23106 & J23119) of the Anderson family. The substrate (nitrocefin) hydrolysis (490nm) is divided by cell growth (600nm), in order to normalize all values.
The maximum expression of β-lactamase was observed under control of the J23119 (brown line) which is the wild type promoter of the Anderson family. The expression is reduced with the J23100 and J23106 (yellow and purple line respectively), while the lowest expression levels were observed with the J23105 promoter (blue line). These results are in accordance with those from previous teams that measured fluorescence and the same pattern is observed. The controls conditions (pSB1C3 and BL21, or light purple and light blue respectively) confirm that the absorbance measured derives from β-lactamase activity only, both quantitatively and visually.
Below you can see the 96-well plate of the assay:

Figure 3.The observed color change due to the hydrolyzation of nitrocefin due to the production of β-lactamase, after a 2-hour enzymatic assay.
Note that the picture was taken after the plate-reader assay was completed and all conditions had reached a plateau, except the controls.
User Reviews
UNIQ2de92f4ebd6afd62-partinfo-0000000A-QINU UNIQ2de92f4ebd6afd62-partinfo-0000000B-QINU The lactamases shows great activity at 37°C and 30°C incubation over night on agar plates. The transformed E.coli cells were able to survive with ampicillin concentrations up to 400 µg/ml at 37°C and at 30°C.
