Difference between revisions of "Part:BBa K1362400"
| (12 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K1362400 short</partinfo> | <partinfo>BBa_K1362400 short</partinfo> | ||
| − | + | <i>Npu</i>DnaE split Intein, N-terminal half. This is a DNA piece for cloning used to assemble other BioBrick parts. | |
| + | |||
| + | ===<i>Nostoc punctiforme</i> DnaE split intein (<i>Npu</i> DnaE)=== | ||
| + | Inteins can be found in all kingdoms of life, however their use for the organism remains yet unknown. In the past years many intein sequences were found in the genomes of cyanobacteria. | ||
| + | <i>Npu</i> DnaE is a naturally split intein from the alpha subunit of Dna Polymerase III in Nostoc punctiforme bacteria which shows [[{{PAGENAME}}:Design#References|[1]]] extraordinarly high splicing activity and therefore is currently still on of the gold-standards for inteins. | ||
| + | |||
| + | <pre> | ||
| + | Amino Acid Sequence: | ||
| + | |||
| + | N-Intein: AEY | CLSYETEILTVEYGLLPIGKIVEKRIECTVYSVDNNGNIYTQPVAQWHDRGEQEVFEYCLEDGSLIRATKDHKFMTVDGQMLPIDEIFERELDLMRVDNLPN | ||
| + | C-Intein: MIKIATRKYLGKQNVYDIGVERDHNFALKNGFIASN | CFN | ||
| + | </pre> | ||
| + | |||
| + | The sequences above are specified with three native extein residues on each of the termini. Please note that in the case of <i>Npu</i> DnaE these are not the most efficient amino acids in supporting the splicing reaction. Detailed information that we found to be very useful is given in [[{{PAGENAME}}:Design#References|[2]]]. | ||
| + | |||
| + | |||
| + | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 11: | Line 26: | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K1362400 SequenceAndFeatures</partinfo> | <partinfo>BBa_K1362400 SequenceAndFeatures</partinfo> | ||
| + | <br> | ||
| + | |||
| + | ===Characterization in <i>Nicotiana benthamiana</i>=== | ||
| + | |||
| + | <b>Group</b>: [http://2016.igem.org/Team:Valencia_UPV Valencia_UPV team 2016] | ||
| + | |||
| + | <b>Author</b>: Valencia UPV iGEM team (Iván Casas Rodrigo, Mónica Victoria Gutiérrez Salazar, Alicia Climent Catalá, Álvaro Ballesteros González) | ||
| + | |||
| + | <b>Summary</b> | ||
| + | |||
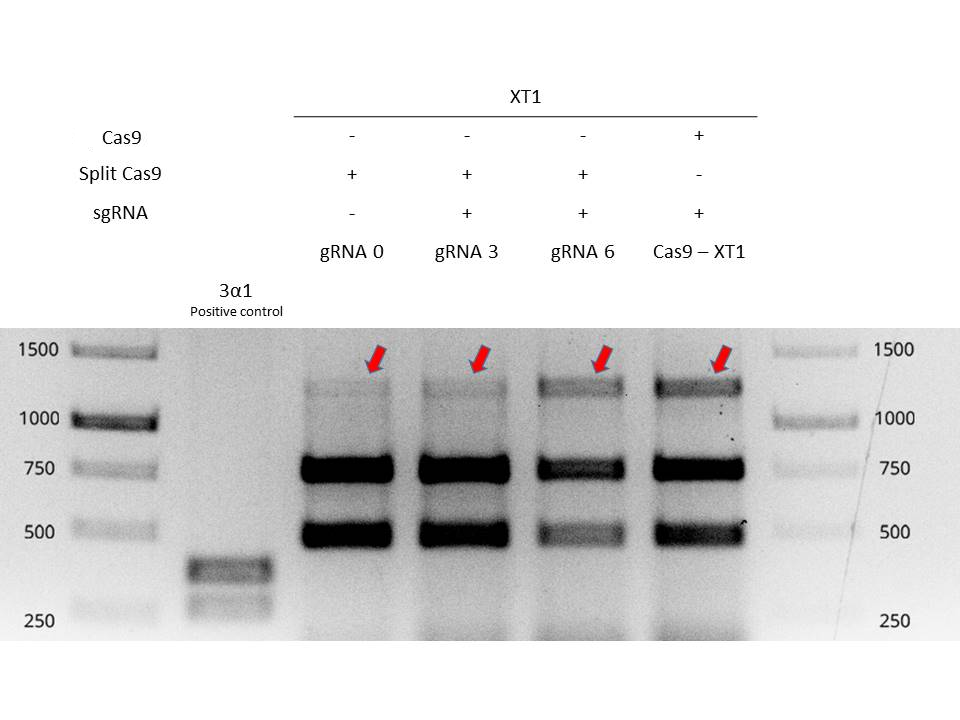
| + | Valencia UPV 2016 team tested and demonstrated the functionality of inteins in a plant chassis, specifically in <i>Nicotiana benthamiana</i>. We used the inteins with our split-Cas9 ([https://parts.igem.org/Part:BBa_K2017000 BBa_K2017000] and [https://parts.igem.org/Part:BBa_K2017001 BBa_K2017001]). The inteins allowed to fusion of the two parts of the split-Cas9 in vivo, in order to obtain a fully functional Cas9 protein. In Figure 1 it is shown an electrophoresis gel with the results of the testing of split-Cas9 in plants. For further information about the experimental design, check the information of split-Cas9 parts, [https://parts.igem.org/Part:BBa_K2017000 BBa_K2017000] and [https://parts.igem.org/Part:BBa_K2017001 BBa_K2017001] | ||
| + | |||
| + | [[Image:BBa_K201700_digestionsplitcas9.jpg|600px|thumb|center|'''Figure 1:''' Electrophoresis gel of XT1 amplicons digestion. Mutation efficiencies of Split-intein-Cas9 system and classical Cas9 system are compared by resistant bands signal intensity.]] | ||
| + | |||
| + | |||
| + | ===Information added by Team IISER-Pune-India 2020=== | ||
| + | <b>Team</b>: IISER-Pune-India 2020 | ||
| + | |||
| + | <b>Author</b>: Avadhoot Jadhav | ||
| + | |||
| + | <b>Summary</b> | ||
| + | |||
| + | *We aimed to use this part for circularization of the cyclotide protein. To build the biobrick we preferred restriction-free cloning but we realised that the biobrick was not compatible with restriction-free cloning. | ||
| + | [[Image:1 BBa K1362400.png|750px|thumb|center|Figure 1. Translation product obtained from the C-intein construct using Geneious.]] | ||
| + | In the translation product, we observe that the amino acid sequence does not fully match the claimed sequence. The first few amino acids are missing. We also note that the first few nucleotide sequences are not inframe; meaning that to create a scarless assembly of different components using Gibson' assembly or RF cloning, this part would not be compatible. | ||
| + | So we modified this part and built the modified version of N-Intein which is compatible with restriction-free cloning technique: BBa_K3582022. | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Latest revision as of 16:48, 13 October 2021
NpuDnaE N-Intein cloning piece
NpuDnaE split Intein, N-terminal half. This is a DNA piece for cloning used to assemble other BioBrick parts.
Nostoc punctiforme DnaE split intein (Npu DnaE)
Inteins can be found in all kingdoms of life, however their use for the organism remains yet unknown. In the past years many intein sequences were found in the genomes of cyanobacteria. Npu DnaE is a naturally split intein from the alpha subunit of Dna Polymerase III in Nostoc punctiforme bacteria which shows [1] extraordinarly high splicing activity and therefore is currently still on of the gold-standards for inteins.
Amino Acid Sequence: N-Intein: AEY | CLSYETEILTVEYGLLPIGKIVEKRIECTVYSVDNNGNIYTQPVAQWHDRGEQEVFEYCLEDGSLIRATKDHKFMTVDGQMLPIDEIFERELDLMRVDNLPN C-Intein: MIKIATRKYLGKQNVYDIGVERDHNFALKNGFIASN | CFN
The sequences above are specified with three native extein residues on each of the termini. Please note that in the case of Npu DnaE these are not the most efficient amino acids in supporting the splicing reaction. Detailed information that we found to be very useful is given in [2].
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterization in Nicotiana benthamiana
Group: [http://2016.igem.org/Team:Valencia_UPV Valencia_UPV team 2016]
Author: Valencia UPV iGEM team (Iván Casas Rodrigo, Mónica Victoria Gutiérrez Salazar, Alicia Climent Catalá, Álvaro Ballesteros González)
Summary
Valencia UPV 2016 team tested and demonstrated the functionality of inteins in a plant chassis, specifically in Nicotiana benthamiana. We used the inteins with our split-Cas9 (BBa_K2017000 and BBa_K2017001). The inteins allowed to fusion of the two parts of the split-Cas9 in vivo, in order to obtain a fully functional Cas9 protein. In Figure 1 it is shown an electrophoresis gel with the results of the testing of split-Cas9 in plants. For further information about the experimental design, check the information of split-Cas9 parts, BBa_K2017000 and BBa_K2017001
Information added by Team IISER-Pune-India 2020
Team: IISER-Pune-India 2020
Author: Avadhoot Jadhav
Summary
- We aimed to use this part for circularization of the cyclotide protein. To build the biobrick we preferred restriction-free cloning but we realised that the biobrick was not compatible with restriction-free cloning.
In the translation product, we observe that the amino acid sequence does not fully match the claimed sequence. The first few amino acids are missing. We also note that the first few nucleotide sequences are not inframe; meaning that to create a scarless assembly of different components using Gibson' assembly or RF cloning, this part would not be compatible. So we modified this part and built the modified version of N-Intein which is compatible with restriction-free cloning technique: BBa_K3582022.