Difference between revisions of "Part:BBa E1010:Experience"
(→User Reviews) |
Kristiturton (Talk | contribs) (→Lethbridge_HS iGEM 2019) |
||
| (46 intermediate revisions by 9 users not shown) | |||
| Line 2: | Line 2: | ||
This experience page is provided so that any user may enter their experience using this part.<BR>Please enter | This experience page is provided so that any user may enter their experience using this part.<BR>Please enter | ||
how you used this part and how it worked out. | how you used this part and how it worked out. | ||
| + | |||
| + | |||
| + | |||
| + | ===RFC25 compatible version of this BioBrick is available=== | ||
| + | |||
| + | The iGEM-Team iGEM14_LMU-Munich did two silent mutations to delete the two AgeI restriction sites within this BioBrick in order to gain a RFC25 compatible version of the BioBrick BBa_E1010. | ||
| + | This new BioBrick is called [https://parts.igem.org/Part:BBa_K135102 BBa_K1351021] | ||
| + | |||
===RANDOM SEQUENCE FOUND WITHIN PART=== | ===RANDOM SEQUENCE FOUND WITHIN PART=== | ||
| Line 7: | Line 15: | ||
*Please note that the above sequence is the old "barcode" sequence added to all of the original CDSs in the early BioBrick part collections. I.e., it's not a random sequence. See https://parts.igem.org/cgi/htdocs/barcodes.cgi for more information (D. Endy). | *Please note that the above sequence is the old "barcode" sequence added to all of the original CDSs in the early BioBrick part collections. I.e., it's not a random sequence. See https://parts.igem.org/cgi/htdocs/barcodes.cgi for more information (D. Endy). | ||
**'''FURTHER NOTE''' The Registry is not displaying barcodes on any of the original parts. The presented sequence information is wrong. This is a serious bug in the Registry that need to be fixed (D. Endy). [[User:Drew|Drew]] 14:34, 1 November 2010 (UTC) | **'''FURTHER NOTE''' The Registry is not displaying barcodes on any of the original parts. The presented sequence information is wrong. This is a serious bug in the Registry that need to be fixed (D. Endy). [[User:Drew|Drew]] 14:34, 1 November 2010 (UTC) | ||
| + | |||
| + | ===Addition of His tag: Lethbridge 2019=== | ||
| + | |||
| + | For the purposes of our project we have added a his tag on the mRFP1 sequence for purification reasons. Although we have not been able to characterize just the His-mRFP1 construct this year ( composite parts [https://parts.igem.org/Part:BBa_K3237014 BBa_K3237014] and [https://parts.igem.org/Part:BBa_K3237015 BBa_K3237015]), we have used this fused to pro insulin and SCI57 insulin variants in our project. Our purification data can be found on our website [https://2019.igem.org/Team:Lethbridge/Results Lethbridge iGEM 2019] or on parts pages [https://parts.igem.org/Part:BBa_K3237016 BBa_K3237016] and [https://parts.igem.org/Part:BBa_K3237018 BBa_K3237018]. These fusions were also designed for use in <i> chlamydomonas reinhardtii </i>. For more information see our parts [https://parts.igem.org/Part:BBa_K3237017 BBa_K3237017] and [https://parts.igem.org/Part:BBa_K3237019 BBa_K3237019]. | ||
| + | |||
| + | |||
| + | ===Lethbridge_HS iGEM 2019=== | ||
| + | |||
| + | Our team utilized this part to characterize the therapeutic portion of our CRISPR Cas13a system. In brief, we designed CRISPR RNAs (crRNA) to target a portion of the GFP mRNA transcript that interact with four different Cas13a proteins from four organisms. We used RFP as a specificity control to ensure that our crRNAs would not cleave any RFP transcripts. In theory, we had hoped that all three plasmids (GFP or RFP, crRNA, and Cas13a) would be transformed into <i> E. coli </i> Rosetta(DE3) cells to determine if we could have specific targeting of GFP <i> in vivo </i>. While we were unable to get our triple plasmid transformation to work, we were able to add characterization data related to the growth and fluorescence of RFP in <i> E. coli </i> DH5α cells, and in a dual plasmid transformation into <i> E. coli </i> BL21(DE3) cells with RFP and the crRNA plasmid. We continued to work with RFP in the plasmid pSB1C3. | ||
| + | |||
| + | Initially, we worked on characterizing how RFP is able to grow independently. Our team wanted to create a standardized fluorescence curve for RFP as well as a standardized growth curve for the <i>E. coli</i> DH5α cells expressing it. Then, when it came time to conduct our <i>in vivo</i> fluorescence assay, these curves would prove to be useful references for comparison with our results. We pipetted biological replicates of RFP into a 96 well plate, then used a spectrophotometer to observe optical density over time, and also a spectrometer to observe fluorescence over time. Figure 1 showed what we had expected the growth to be with an increase in doubling time likely due to the load placed on the cell from expressing the plasmid. Figure 1 showed what we had expected the growth to be with an increase in doubling time likely due to the load placed on the cell based on the plasmid. However, further attempts at replicating this data have been unsuccessful (figure 2). We are uncertain as to the cause of this issue. We suspect that by using overnight cultures, the fluorescent protein may already be matured and thus we did not get reliable fluorescence data or the protein did not fold properly <i> in vivo </i>. | ||
| + | |||
| + | |||
| + | https://2019.igem.org/wiki/images/c/ce/T--Lethbridge_HS--jul_od_single.png | ||
| + | <b> Figure 1. </b> Growth of <i>Escherichia coli</i> DH5α cells containing GFP or RFP. 3 biological replicates for each fluorescent protein and 7 technical replicates were completed. Shown are all replicates averaged together and the standard deviation. The <i> E. coli </i> cells expressing GFP average doubling time varied from 38.2 +/- 0.9 minutes to 41.8 +/- 1.6 minutes and for cells expressing RFP the average doubling time varied from 37.8 +/- 0.2 minutes to 41.0 +/- 0.5 minutes. | ||
| + | |||
| + | https://2019.igem.org/wiki/images/8/8f/T--Lethbridge_HS--aug_single_combined.png | ||
| + | <b> Figure 2. </b> Growth of <i>Escherichia coli</i> DH5α cells containing GFP or RFP. 3 biological replicates for each fluorescent protein and 5 technical replicates were completed. Shown are the average technical replicates and the standard deviation. <b> (A) </b> Optical density of <i> E. coli </i> cells expressing GFP or RFP with absorbance measured at 600 nm. <b> (B) </b> Fluorescence of <i> E. coli </i> cells expressing GFP or RFP. GFP excitation was measured at 475 nm and emission at 508 nm. RFP excitation was measured at 558 nm and emission at 583 nm. | ||
| + | |||
| + | Figure 3 shows a co-culture assay with a 1:1 ratio of GFP and RFP expressed in <i> E. coli </i>. We wanted to see if one culture of fluorescent protein would outcompete the other present. As with the single cultures shown in figure 2, the cells replicated slowly. Our fluorescence data indicated that the GFP we are using is more fluorescent than RFP. The fluorescence of RFP also showed greater variation, indicating that the RFP may not be folding properly to be excited in all replicates. | ||
| + | |||
| + | https://2019.igem.org/wiki/images/3/33/T--Lethbridge_HS--aug_co-culture_combined.png | ||
| + | <b> Figure 3. </b>Co-culture of <i> Escherichia coli </i> DH5α cells containing GFP or RFP. Equal amounts of each cell type were added into a 96 well plate and grown together to see how they would grow in tandem. 3 biological replicates for each fluorescent protein and 6 technical replicates were completed. Shown are all replicates averaged together and the standard deviation. <b> (A) </b> Optical density of <i> E. coli </i> cells expressing GFP or RFP with absorbance measured at 600 nm.<b> (B) </b> Fluorescence of <i> E. coli </i> cells expressing GFP or RFP. GFP excitation was measured at 475 nm and emission at 508 nm. RFP excitation was measured at 558 nm and emission at 583 nm. | ||
| + | |||
| + | We completed a dual plasmid transformation. To confirm that we had both plasmids present we completed a digest test because PCR amplification from the colony was not working. This should separate our construct from the plasmid confirming that both parts are present. In figure 4, only linearized cuts are seen. Due to both plasmids being approximately the same size it is difficult to see. However, there are two bands present. | ||
| + | |||
| + | Initially, our team had planned on doing a triple plasmid transformation of our target fluorescent protein (GFP), crRNA, and Cas13a to test if our system would work <i> in vivo </i>. Additionally, we wanted to have RFP as the fluorescent protein to serve as a specificity control instead of transforming the plasmid containing GFP. We were unsuccessful in getting all three plasmids to transform, but did succeed in getting the fluorescent proteins and crRNA containing plasmids to transform together as seen in figure 4. | ||
| + | |||
| + | https://2019.igem.org/wiki/images/5/55/T--Lethbridge_HS--rfp.png | ||
| + | <b> Figure 4. </b> 1% agarose of restriction digests using PstI and EcoRI from <i> Escherichia coli </i> BL21(DE3) cells containing plasmids for RFP and either Lbu crRNA or Lwa crRNA. Left to right: lane 1: 1 kb ladder; lane 2: Lwa crRNA colony 1; lane 3: Lwa crRNA colony 2; lane 4: Lwa crRNA colony 3; lane 5: Lbu crRNA colony 1; lane 6: Lbu crRNA colony 2; lane 7: Lbu crRNA colony 3; lanes 8-13: empty. | ||
| + | |||
| + | As an alternative experiment, our team grew cells that expressed the Lbu and Lwa Cas13a protein overnight. We also grew cells that expressed dual plasmids; GFP and crRNA Lwa; GFP and crRNA Lbu; RFP and crRNA Lwa; and RFP and crRNA Lbu. We then lysed the cells that expressed the Cas13a proteins using a French Press and clarified the lysate via centrifugation. Following this, our team pipetted in a 1:1 ratio of clarified cell lysate: fluorescent protein and crRNA into a 96 well plate. This allowed us to observe if there would be an effect from the CRISPR Cas13a system on the fluorescent proteins. We observed that in our optical density data, both dual plasmid systems for GFP and RFP had stunted growth in comparison to only <i> E. coli </i> cells expressing GFP or RFP or no plasmid (Figure 5C). Adding the lysate may have caused the death of the culture. We neglected to include replicates of the dual plasmid system without adding lysate to observe how that grew. This would be beneficial for any future experiments. Alternatively, there may have been some effect of the protein in the lysate on the GFP fluorescence (Figure 5B). However, we are unsure of the specificity due to the potential of the RFP not folding correctly <i>in vivo </i> as demonstrated by the substantial standard deviation seen in our replicates (Figure 5A). | ||
| + | |||
| + | https://2019.igem.org/wiki/images/9/99/T--Lethbridge_HS--invivo_fluor.png | ||
| + | <b> Figure 5 </b> <i> In vivo</i> fluorescence assay of <i> E. coli </i> BL21(DE3) cells containing fluorescent protein and crRNA plasmids and <i> E. coli </i> Rosetta(DE3) cell lysate of overexpressed Cas13a proteins. This assay was conducted with 3 biological replicates and 3 technical replicates. <b> (A) </b> Fluorescence of <i> E. coli </i> cells containing only an RFP expressing plasmid, or dual plasmid expression of RFP and crRNAs from Lwa and Lbu that target GFP and the respective cell lysate containing the appropriate Cas13a. RFP excitation was at 558 nm and emission at 583 nm. <b> (B) </b> Fluorescence of <i> E. coli </i> cells containing only a GFP expressing plasmid, or dual plasmid expression of GFP and crRNAs from Lwa and Lbu that target GFP and the respective cell lysate containing the appropriate Cas13a. GFP excitation was at 475 nm and emission at 508 nm.<b> (C)</b> Optical density of <i> E. coli </i> cells expressing GFP, RFP, dual plasmid systems mentioned previously, or only <i> E. coli </i> BL21(DE3) cells with absorbance measured at 600 nm.<b> (D)</b> Relative fluorescence at maximum excitation at 81 minutes. GFP excitation was at 475 nm and emission at 508 nm and RFP excitation was at 558 nm and emission at 583 nm. | ||
===Applications of BBa_E1010=== | ===Applications of BBa_E1010=== | ||
| + | |||
| + | [[File:Example.jpg|400px]] <!--Be sure to size your images for proper formatting --> | ||
| + | |||
| + | |||
| + | <hr> | ||
| + | <p> | ||
| + | '''iGEM14_Carnegie_Mellon'''. | ||
| + | We characterized a set of fluorescent proteins consisting of BFP. GFP, YFP, OFP, and RFP. We calculated the signal-to-noise ratio of all the proteins in two different cell lines (MACH and Top10). RFP had a high signal-to-noise ratio in both cell lines. RFP was measured at (ex/em = 584nm/607nm). | ||
| + | </p> | ||
| + | https://static.igem.org/mediawiki/2014/thumb/c/c1/FP_MACH.png/591px-FP_MACH.png | ||
| + | https://static.igem.org/mediawiki/2014/thumb/9/9f/Top10_Best.jpg/591px-Top10_Best.jpg | ||
| + | |||
===User Reviews=== | ===User Reviews=== | ||
| Line 22: | Line 77: | ||
|}; | |}; | ||
<!-- End of the user review template --> | <!-- End of the user review template --> | ||
| + | {|width='80%' style='border:1px solid gray' | ||
| + | |- | ||
| + | |width='10%'| | ||
| + | <partinfo>BBa_E1010 AddReview 4</partinfo> | ||
| + | <I>iGEM Kyoto 2018</I> | ||
| + | |width='60%' valign='top'| | ||
| + | '''Characterization of RFP BBa_E1010'''<br> | ||
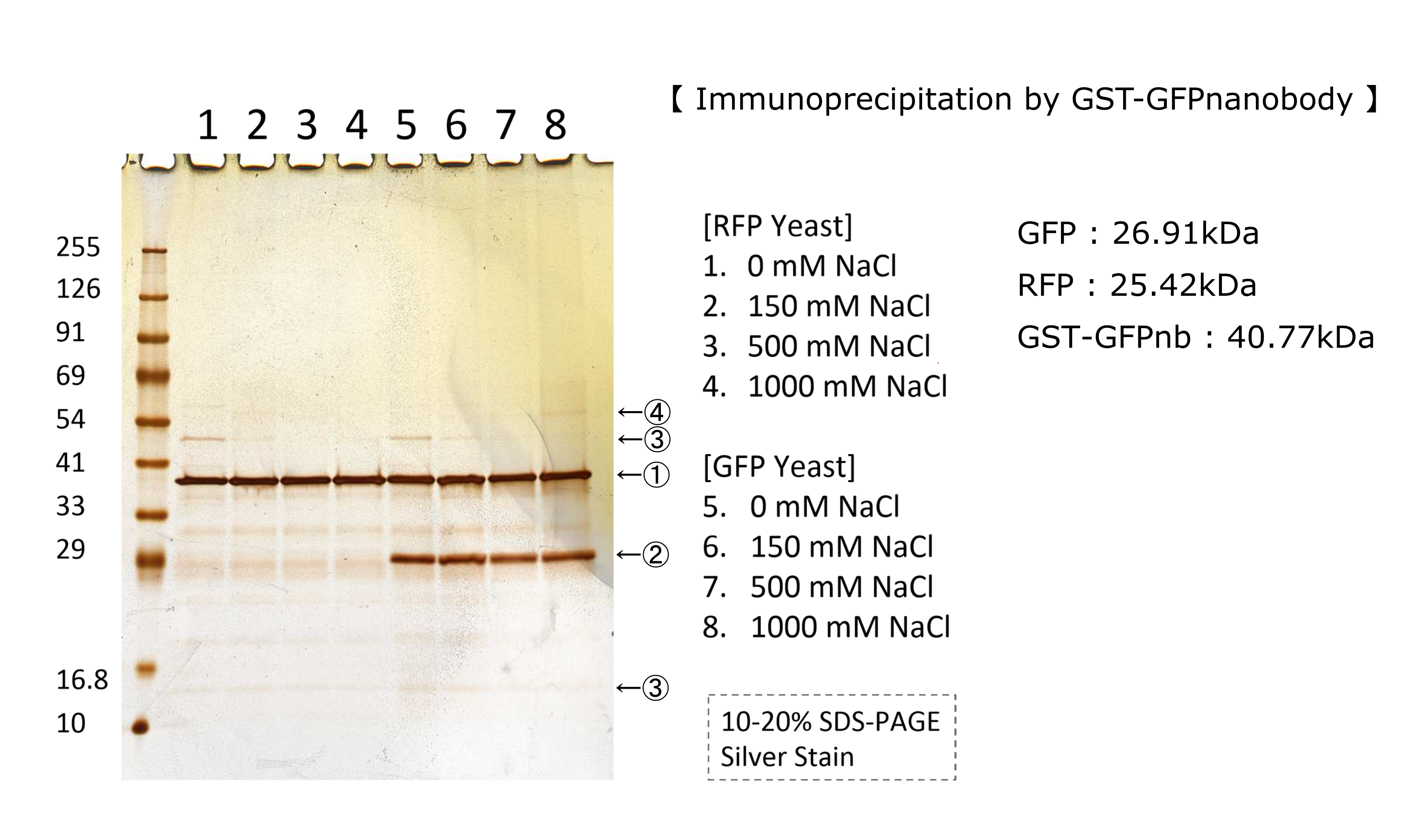
| + | We have characterized RFP BBa_E1010 in several ways.<br> | ||
| + | At first,we did immunoprecipitation by GFP nanobody in various salt concentration.Figure below shows RFP does not combine with GFPnanobody.Detail of this experiment is in this page''' [http://2018.igem.org/Team:Kyoto/Result Kyoto 2018] ''' | ||
| + | [[File:T--Kyoto--GFP IP.png|700px]] | ||
| + | Secondly,TDH3 promoter and CYC1 terminator were added to both ends of ORF and cloned into pRS316 which is a shuttle vector of S. cerevisiae and E. coli. The resulting plasmid was transformed into wild-type yeast strain BY 4741 to overexpress mRFP in yeast.<br> | ||
| + | Photographs of pellets recovered from the culture medium of yeast cells is below.As can be easily seen, the yeast pellet overexpressing RFP was colored in a thin red color.From this, it was confirmed that RFP of BBa_E0010 can be expressed in large amounts in yeast cells without changing the codon and that the expression level thereof is so large as to be visually observed under visible light without breaking the yeast.<br> | ||
| + | [[File:T--Kyoto--Yeast GFPRFP.jpeg|300px]]<br> | ||
| + | Additionally, we captured RFP expressing cells that has FLAG tag on surface with magnetic beads coated with anti-Flag antibody. | ||
| + | We can see strong fluorescence even in a single yeast.<br> | ||
| + | [[File:T--Kyoto--FgβF3-FLAG.jpeg|500px|Figure 1]] <br> | ||
| + | |}; | ||
| + | |||
| + | |||
| + | {|width='80%' style='border:1px solid gray' | ||
| + | |- | ||
| + | |width='10%'| | ||
| + | <partinfo>BBa_E1010 AddReview 5</partinfo> | ||
| + | <I>Hong Kong-CUHK iGEM 2017</I> | ||
| + | |width='60%' valign='top'| | ||
| + | |||
| + | <b>Charaterization of mRFP pH stabillity</b> | ||
| + | <p>We grew C41 bacteria with parts BBa_J61002 in 2XYT for 24 hours. Purifying the mRFP by Ion Exchange Chromatography and Hydrophobic Interaction Chromatography, we measured the fluoresece (ex ,em ) of purified mRFP. which is diluted to 10µg/100µl (total 200µl) in triplicates, into different buffers (ranges from pH2 to pH12). The result shows that the stability drops dramatically in pH condition below 6 and relatively stable in pH 6-10. </p> | ||
| + | <div align="center"> | ||
| + | |||
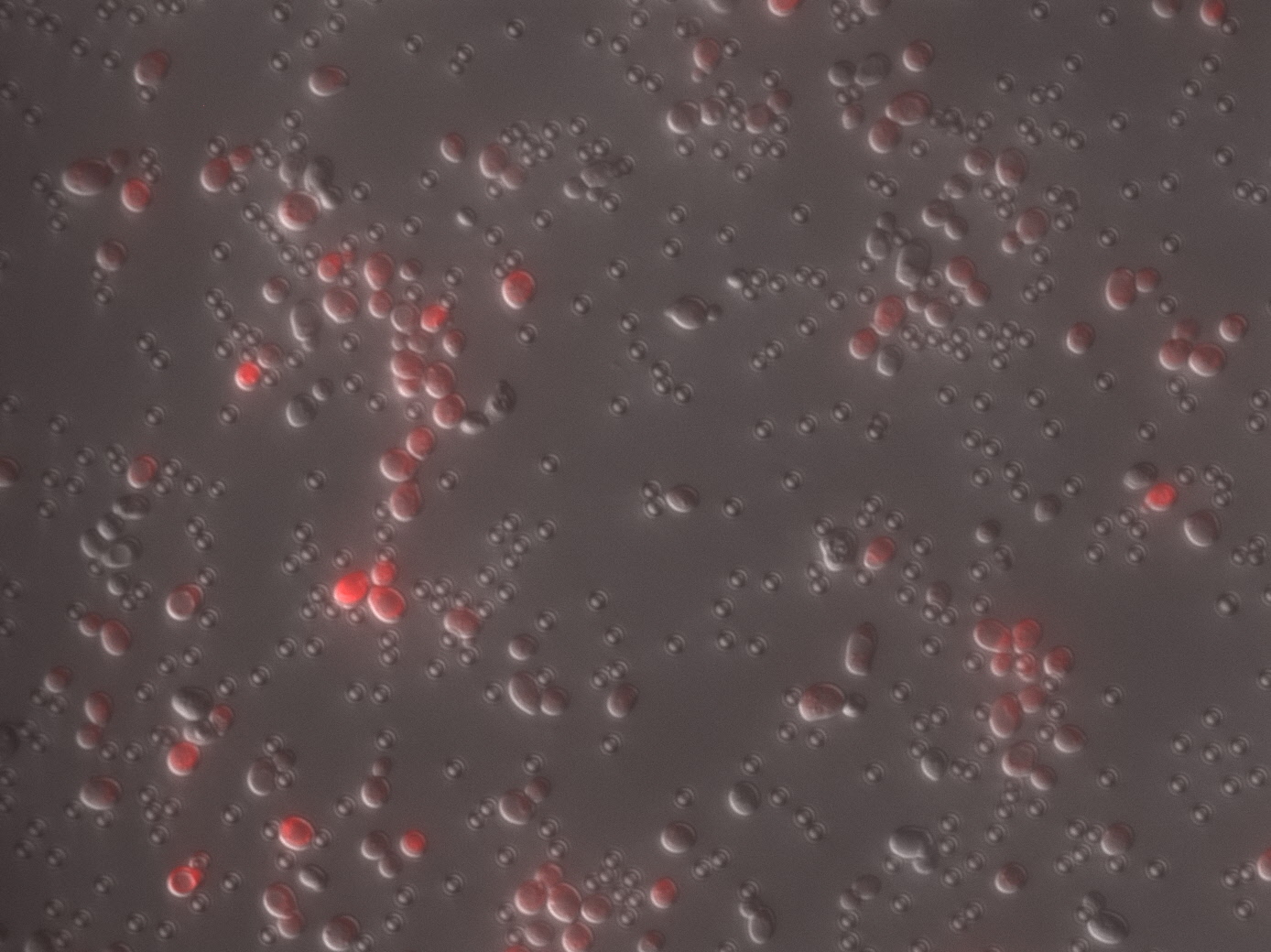
| + | [[File:Mrfp.PNG|center|thumb|350px|''<b>Fig.1</b> Vary pH attributed to different fluorescent intensity of RFP.]] | ||
| + | <table cellpadding="2" border="1px" cellspacing="0" align="center" width="70%"> | ||
| + | <caption><p align="justify"><b>Table 1</b> Plate reader setting of fluorescent measurement</p></caption> | ||
| + | <td><b>Measurement Type</b></td> | ||
| + | <td>Fluorescence</td> | ||
| + | |||
| + | <tr> | ||
| + | <td><b>Microplate name<</b>/td> | ||
| + | <td>COSTAR 96</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><b>Scan mode</b></td> | ||
| + | <td>orbital averaging</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><b>Scan diameter [nm]</b></td> | ||
| + | <td>3</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><b>Excitation</b></td> | ||
| + | <td>550-20</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><b>Emission</b></td> | ||
| + | <td>605-40</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><b>Dichronic filter</b> </td> | ||
| + | <td>auto 572.5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><b>Gain </b></td> | ||
| + | <td>500</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><b>Focal height [nm]</b></td> | ||
| + | <td>9</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | |}; | ||
| + | |||
| + | {|width='80%' style='border:1px solid gray' | ||
| + | |- | ||
| + | |width='10%'| | ||
| + | <partinfo>BBa_E1010 AddReview 5</partinfo> | ||
| + | <I>Immudzen</I> | ||
| + | |width='60%' valign='top'| | ||
| + | |||
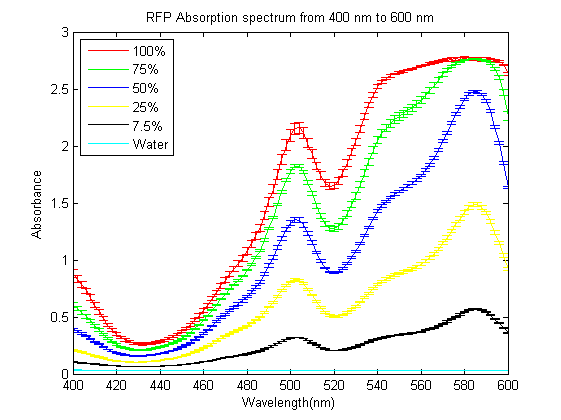
| + | As part of the 2013 CU Boulder project we worked on separating RFP from AmilCP and during that process we ran into a problem that the fluorescence of RFP is too close to the absorption of AmilCP to tell them apart. What we ended up doing was measuring the spectrum of RFP from 400nm to 600nm. | ||
| + | |||
| + | [[File:Cu_RFP.png]] | ||
| + | |||
| + | What we found is that RFP has a secondary absorbance peak at 502nm which is well clear of AmilCP. Under the devices we had access to this secondary peak also remained in the linear region of our device over a much wider concentration range. | ||
| + | |||
| + | We also found that when running on an agarose gel RFP will run down on the gel while AmilCP runs up on the gel. | ||
| + | |}; | ||
| + | |||
| + | {|width='80%' style='border:1px solid gray' | ||
| + | |- | ||
| + | |width='10%'| | ||
| + | <partinfo>BBa_E1010 AddReview 5</partinfo> | ||
| + | <I>HIT-Harbin</I> | ||
| + | |width='60%' valign='top'| | ||
| + | <p></p>1)Measuring absorbance of RFP | ||
| + | <p>We grew bacteria without device(BBa_J04450) and bacteria with parts BBa_J04450 in | ||
| + | |||
| + | same volume until stationary phase. Taking bacteria without device as background, we | ||
| + | |||
| + | measured the absorbance of bacteria with our device (the max absorption peak is | ||
| + | |||
| + | 504nm).But absorbance in 504nm is higher than 1,which present a bad linear relation | ||
| + | |||
| + | between absorbance and concentraton. RFP has absorption in 450nm,and absorbance is | ||
| + | |||
| + | between 0.1 and 1(better linear relation).Occasionally, we find a RFP standard curve | ||
| + | |||
| + | under 450nm on the web.Before the mensuration, we diluted the two groups according to | ||
| + | |||
| + | table1. We took the mean of two measures as the useful data. </p> | ||
| + | <div align="center"> | ||
| + | "https://static.igem.org/mediawiki/2013/8/8f/Experiment8.png" | ||
| + | <p>Fig.1 RFP absorbance varying with wave length</p> | ||
| + | <p>Table 1 Dilution of Two groups of bacteria</p> | ||
| + | "https://static.igem.org/mediawiki/2013/2/21/Experiment7.png" | ||
| + | "https://static.igem.org/mediawiki/2013/7/74/Experiment9.png" | ||
| + | <p>Fig 2. The relationship between RFP concentration and absorbance(OD450)</p> | ||
| + | </div> | ||
| + | |||
| + | <p>2)The actual relationship between RFP concentration and absorbance</p> | ||
| + | <div align="center"> | ||
| + | "https://static.igem.org/mediawiki/2013/8/88/Experiment10.png" | ||
| + | <p>Fig 3. RFP standard curve obtain from the web</p> | ||
| + | </div> | ||
| + | <p></p>Through the standard curve, we can convert the relative concentration to the | ||
| + | |||
| + | absolute concentration, and finally get the relationship between IPTG concentration | ||
| + | |||
| + | and RFP concentration. | ||
| + | <p></p>Compared to crushing cells to separate RFP, our method is simpler and easy to | ||
| + | |||
| + | practice. Moreover, our relative concentration curve is credible. If the standard | ||
| + | |||
| + | curve is reliable, our calculated result of RFP will be precise. | ||
| + | |||
| + | |}; | ||
| + | |||
| + | {|width='80%' style='border:1px solid gray' | ||
| + | |- | ||
| + | |width='10%'| | ||
| + | <partinfo>BBa_E1010 AddReview 5</partinfo> | ||
| + | <I>Carnegie_Mellon 2013</I><br /> | ||
| + | |||
| + | |width='60%' valign='top'| | ||
| + | <p align="center"><b> Characterization of the Photostability of mRFP1</b></p> | ||
| + | [[file: MRFP1.png |center|thumb|400px|''Photobleaching curve of mRFP1 with a HBO100 mercury-arc lamp"]] | ||
| + | XL10 Ultracompetent cells were transformed with <partinfo>BBa_E1010</partinfo> cloned with <partinfo>BBa_B0034</partinfo> as the RBS and <partinfo>BBa_R0010</partinfo> as the wild-type lac promoter and induced overnight with IPTG.The overnight was bleached for 180 minutes with HBO100 (100W Mercury-arc lamp). Fluorescence data was taken using a Tecan Safire II with the parameters shown in Table 3. Fluorescence values are shown in Table 4. <br /> | ||
| + | <table cellpadding="2" border="1px" cellspacing="0" align="center" width="70%"> | ||
| + | <caption><p align="justify"><b>Table 3</b> Tecan Safire II Parameters</p></caption> | ||
| + | <tr><td><b>Excitation (nm)</b></td><td>585</td></tr> | ||
| + | <tr><td><b>Emission (nm)</b></td><td>610</td></tr> | ||
| + | <tr><td><b>Excitation bandwidth (nm)</b></td><td>10</td></tr> | ||
| + | <tr><td><b>Emission bandwidth (nm)</b></td><td>10</td></tr> | ||
| + | <tr><td><b>Gain</b></td><td>63</td></tr> | ||
| + | <tr><td><b>Number of reads</b></td><td>10</td></tr> | ||
| + | <tr><td><b>Integration Time (microseconds)</b></td><td>40</td></tr> | ||
| + | </table> | ||
| + | <br /> | ||
| + | <table cellpadding="2" border="1px" cellspacing="0" align="center" width="50%"> | ||
| + | <caption><p align="justify"><b>Table 4</b> Shows the fluorescence data over time during photobleaching.</p></caption> | ||
| + | <tr><td align="center"><b>Time (minutes)</b></td><td align="center"><b>Fluorescence (RFU)</b></td> | ||
| + | <tr><td align="left">0</td><td align="right">48694</td></tr> | ||
| + | <tr><td align="left">20</td><td align="right">43083</td></tr> | ||
| + | <tr><td align="left">40</td><td align="right">36842</td></tr> | ||
| + | <tr><td align="left">60</td><td align="right">30239</td></tr> | ||
| + | <tr><td align="left">80</td><td align="right">31281</td></tr> | ||
| + | <tr><td align="left">100</td><td align="right">25273</td></tr> | ||
| + | <tr><td align="left">120</td><td align="right">21467</td></tr> | ||
| + | <tr><td align="left">140</td><td align="right">18081</td></tr> | ||
| + | <tr><td align="left">160</td><td align="right">15251</td></tr> | ||
| + | <tr><td align="left">180</td><td align="right">14427</td></tr> | ||
| + | </table> | ||
| + | |} | ||
{|width='80%' style='border:1px solid gray' | {|width='80%' style='border:1px solid gray' | ||
| Line 30: | Line 258: | ||
|width='60%' valign='top'| | |width='60%' valign='top'| | ||
[[image: KAIST_iGEM_2012_Experience_BBa_E1010.PNG|center|thumb|400px|'''Figure 1. E.coli strain MG1655 expressing BBa_E1010 under control of <partinfo>BBa_K907005</partinfo> after overnight culture. 3mL culture with M9 media in 14ml round bottom tube(left), and centrifuged cells in eppendorf tube(right).''' The expression of BBa_1010 is clearly observed with naked eye after overnight culture.]] | [[image: KAIST_iGEM_2012_Experience_BBa_E1010.PNG|center|thumb|400px|'''Figure 1. E.coli strain MG1655 expressing BBa_E1010 under control of <partinfo>BBa_K907005</partinfo> after overnight culture. 3mL culture with M9 media in 14ml round bottom tube(left), and centrifuged cells in eppendorf tube(right).''' The expression of BBa_1010 is clearly observed with naked eye after overnight culture.]] | ||
| + | <br>We recommend you to measure the emission wavelenth at 619nm. Because the maximum excitaion and emission wavelenth are too close to each other, the signal overflows. You can get more precise results with our recommendations. | ||
<br><partinfo>BBa_E1010</partinfo> was successfully used to produce mRFP in E.coli strain MG1655 in LB or M9 minimal media under the control of promoter-<partinfo>BBa_J23119</partinfo> and RBS-<partinfo>BBa_B0034</partinfo> in the Dual Phase Protein Generator(mRFP default), <partinfo>BBa_K907005</partinfo> | <br><partinfo>BBa_E1010</partinfo> was successfully used to produce mRFP in E.coli strain MG1655 in LB or M9 minimal media under the control of promoter-<partinfo>BBa_J23119</partinfo> and RBS-<partinfo>BBa_B0034</partinfo> in the Dual Phase Protein Generator(mRFP default), <partinfo>BBa_K907005</partinfo> | ||
| Line 181: | Line 410: | ||
|- | |- | ||
|width='10%'| | |width='10%'| | ||
| − | <partinfo>BBa_E1010 AddReview | + | <partinfo>BBa_E1010 AddReview 0</partinfo> |
<I>Nkessler</I> | <I>Nkessler</I> | ||
|width='60%' valign='top'| | |width='60%' valign='top'| | ||
We successfully used this part for a read out system, ''e.g.'' in <partinfo>K389016</partinfo>. Additionally we compared it with a luciferase: <partinfo>K389004</partinfo>. | We successfully used this part for a read out system, ''e.g.'' in <partinfo>K389016</partinfo>. Additionally we compared it with a luciferase: <partinfo>K389004</partinfo>. | ||
|} | |} | ||
| + | |||
<!-- DON'T DELETE --><partinfo>BBa_E1010 EndReviews</partinfo> | <!-- DON'T DELETE --><partinfo>BBa_E1010 EndReviews</partinfo> | ||
Latest revision as of 00:42, 21 October 2019
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
RFC25 compatible version of this BioBrick is available
The iGEM-Team iGEM14_LMU-Munich did two silent mutations to delete the two AgeI restriction sites within this BioBrick in order to gain a RFC25 compatible version of the BioBrick BBa_E1010. This new BioBrick is called BBa_K1351021
RANDOM SEQUENCE FOUND WITHIN PART
CGCTGATAGTGCTAGTGTAGATCGC is found after the RFP stop codon and before the BioBricks suffix. Should not affect transcription or translation of RFP, but good to keep note of it especially in analyzing sequencing results. (KP of siGEM)
- Please note that the above sequence is the old "barcode" sequence added to all of the original CDSs in the early BioBrick part collections. I.e., it's not a random sequence. See https://parts.igem.org/cgi/htdocs/barcodes.cgi for more information (D. Endy).
- FURTHER NOTE The Registry is not displaying barcodes on any of the original parts. The presented sequence information is wrong. This is a serious bug in the Registry that need to be fixed (D. Endy). Drew 14:34, 1 November 2010 (UTC)
Addition of His tag: Lethbridge 2019
For the purposes of our project we have added a his tag on the mRFP1 sequence for purification reasons. Although we have not been able to characterize just the His-mRFP1 construct this year ( composite parts BBa_K3237014 and BBa_K3237015), we have used this fused to pro insulin and SCI57 insulin variants in our project. Our purification data can be found on our website Lethbridge iGEM 2019 or on parts pages BBa_K3237016 and BBa_K3237018. These fusions were also designed for use in chlamydomonas reinhardtii . For more information see our parts BBa_K3237017 and BBa_K3237019.
Lethbridge_HS iGEM 2019
Our team utilized this part to characterize the therapeutic portion of our CRISPR Cas13a system. In brief, we designed CRISPR RNAs (crRNA) to target a portion of the GFP mRNA transcript that interact with four different Cas13a proteins from four organisms. We used RFP as a specificity control to ensure that our crRNAs would not cleave any RFP transcripts. In theory, we had hoped that all three plasmids (GFP or RFP, crRNA, and Cas13a) would be transformed into E. coli Rosetta(DE3) cells to determine if we could have specific targeting of GFP in vivo . While we were unable to get our triple plasmid transformation to work, we were able to add characterization data related to the growth and fluorescence of RFP in E. coli DH5α cells, and in a dual plasmid transformation into E. coli BL21(DE3) cells with RFP and the crRNA plasmid. We continued to work with RFP in the plasmid pSB1C3.
Initially, we worked on characterizing how RFP is able to grow independently. Our team wanted to create a standardized fluorescence curve for RFP as well as a standardized growth curve for the E. coli DH5α cells expressing it. Then, when it came time to conduct our in vivo fluorescence assay, these curves would prove to be useful references for comparison with our results. We pipetted biological replicates of RFP into a 96 well plate, then used a spectrophotometer to observe optical density over time, and also a spectrometer to observe fluorescence over time. Figure 1 showed what we had expected the growth to be with an increase in doubling time likely due to the load placed on the cell from expressing the plasmid. Figure 1 showed what we had expected the growth to be with an increase in doubling time likely due to the load placed on the cell based on the plasmid. However, further attempts at replicating this data have been unsuccessful (figure 2). We are uncertain as to the cause of this issue. We suspect that by using overnight cultures, the fluorescent protein may already be matured and thus we did not get reliable fluorescence data or the protein did not fold properly in vivo .
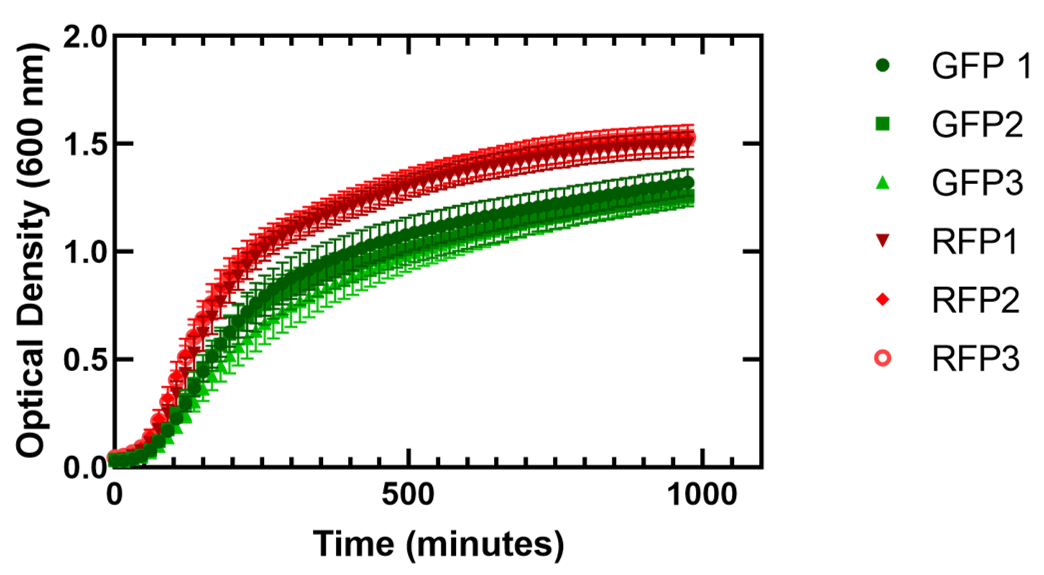 Figure 1. Growth of Escherichia coli DH5α cells containing GFP or RFP. 3 biological replicates for each fluorescent protein and 7 technical replicates were completed. Shown are all replicates averaged together and the standard deviation. The E. coli cells expressing GFP average doubling time varied from 38.2 +/- 0.9 minutes to 41.8 +/- 1.6 minutes and for cells expressing RFP the average doubling time varied from 37.8 +/- 0.2 minutes to 41.0 +/- 0.5 minutes.
Figure 1. Growth of Escherichia coli DH5α cells containing GFP or RFP. 3 biological replicates for each fluorescent protein and 7 technical replicates were completed. Shown are all replicates averaged together and the standard deviation. The E. coli cells expressing GFP average doubling time varied from 38.2 +/- 0.9 minutes to 41.8 +/- 1.6 minutes and for cells expressing RFP the average doubling time varied from 37.8 +/- 0.2 minutes to 41.0 +/- 0.5 minutes.
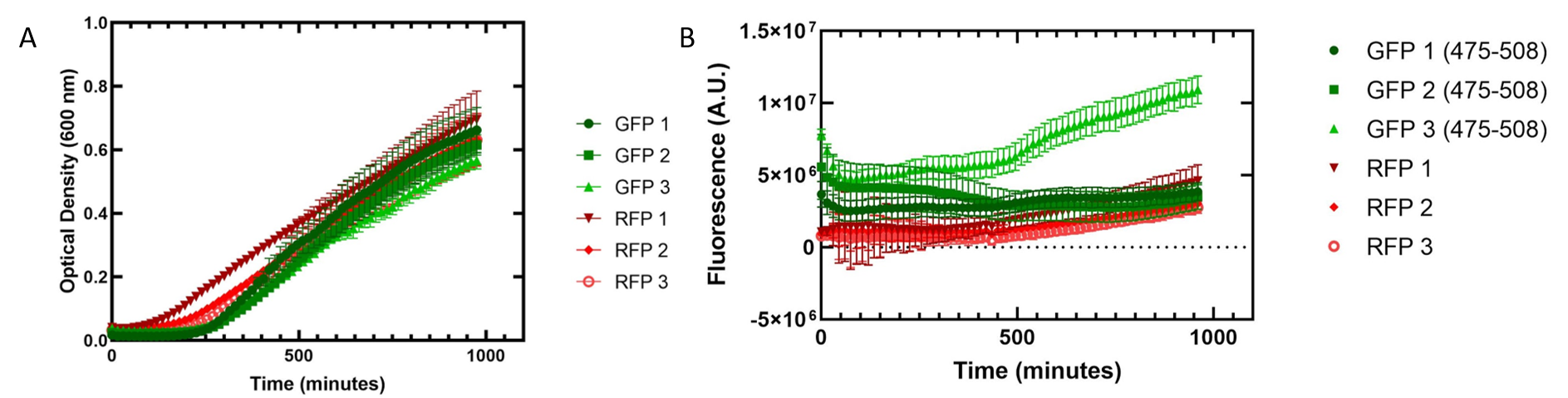 Figure 2. Growth of Escherichia coli DH5α cells containing GFP or RFP. 3 biological replicates for each fluorescent protein and 5 technical replicates were completed. Shown are the average technical replicates and the standard deviation. (A) Optical density of E. coli cells expressing GFP or RFP with absorbance measured at 600 nm. (B) Fluorescence of E. coli cells expressing GFP or RFP. GFP excitation was measured at 475 nm and emission at 508 nm. RFP excitation was measured at 558 nm and emission at 583 nm.
Figure 2. Growth of Escherichia coli DH5α cells containing GFP or RFP. 3 biological replicates for each fluorescent protein and 5 technical replicates were completed. Shown are the average technical replicates and the standard deviation. (A) Optical density of E. coli cells expressing GFP or RFP with absorbance measured at 600 nm. (B) Fluorescence of E. coli cells expressing GFP or RFP. GFP excitation was measured at 475 nm and emission at 508 nm. RFP excitation was measured at 558 nm and emission at 583 nm.
Figure 3 shows a co-culture assay with a 1:1 ratio of GFP and RFP expressed in E. coli . We wanted to see if one culture of fluorescent protein would outcompete the other present. As with the single cultures shown in figure 2, the cells replicated slowly. Our fluorescence data indicated that the GFP we are using is more fluorescent than RFP. The fluorescence of RFP also showed greater variation, indicating that the RFP may not be folding properly to be excited in all replicates.
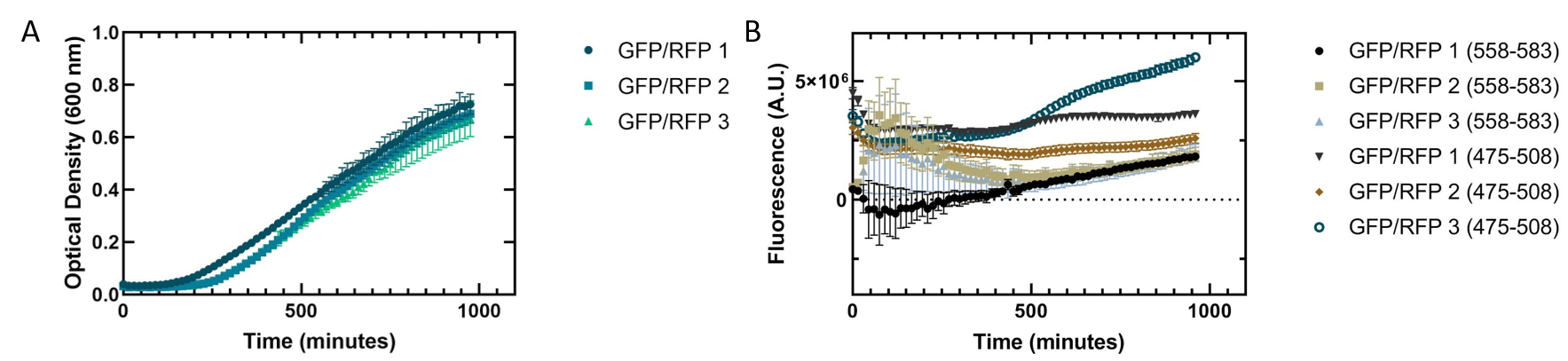 Figure 3. Co-culture of Escherichia coli DH5α cells containing GFP or RFP. Equal amounts of each cell type were added into a 96 well plate and grown together to see how they would grow in tandem. 3 biological replicates for each fluorescent protein and 6 technical replicates were completed. Shown are all replicates averaged together and the standard deviation. (A) Optical density of E. coli cells expressing GFP or RFP with absorbance measured at 600 nm. (B) Fluorescence of E. coli cells expressing GFP or RFP. GFP excitation was measured at 475 nm and emission at 508 nm. RFP excitation was measured at 558 nm and emission at 583 nm.
Figure 3. Co-culture of Escherichia coli DH5α cells containing GFP or RFP. Equal amounts of each cell type were added into a 96 well plate and grown together to see how they would grow in tandem. 3 biological replicates for each fluorescent protein and 6 technical replicates were completed. Shown are all replicates averaged together and the standard deviation. (A) Optical density of E. coli cells expressing GFP or RFP with absorbance measured at 600 nm. (B) Fluorescence of E. coli cells expressing GFP or RFP. GFP excitation was measured at 475 nm and emission at 508 nm. RFP excitation was measured at 558 nm and emission at 583 nm.
We completed a dual plasmid transformation. To confirm that we had both plasmids present we completed a digest test because PCR amplification from the colony was not working. This should separate our construct from the plasmid confirming that both parts are present. In figure 4, only linearized cuts are seen. Due to both plasmids being approximately the same size it is difficult to see. However, there are two bands present.
Initially, our team had planned on doing a triple plasmid transformation of our target fluorescent protein (GFP), crRNA, and Cas13a to test if our system would work in vivo . Additionally, we wanted to have RFP as the fluorescent protein to serve as a specificity control instead of transforming the plasmid containing GFP. We were unsuccessful in getting all three plasmids to transform, but did succeed in getting the fluorescent proteins and crRNA containing plasmids to transform together as seen in figure 4.
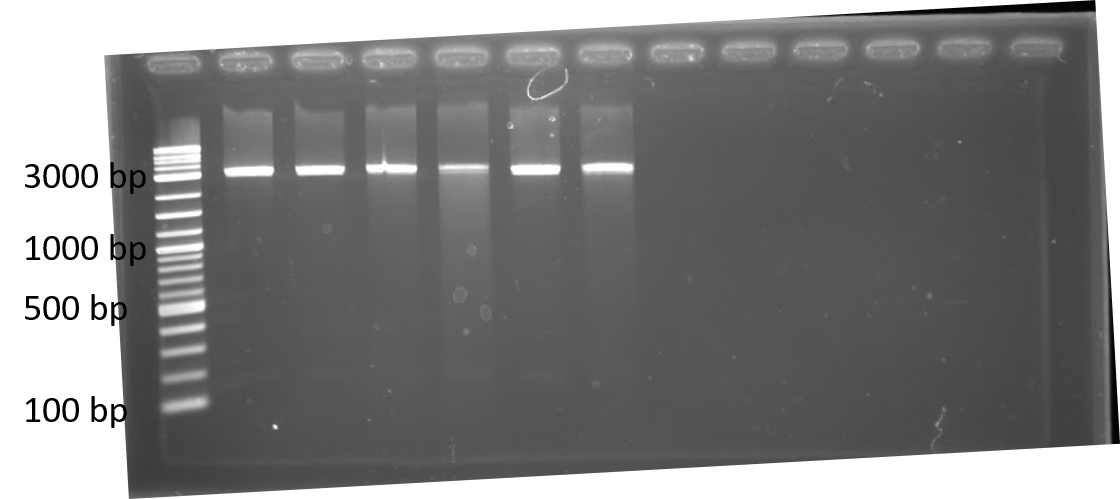 Figure 4. 1% agarose of restriction digests using PstI and EcoRI from Escherichia coli BL21(DE3) cells containing plasmids for RFP and either Lbu crRNA or Lwa crRNA. Left to right: lane 1: 1 kb ladder; lane 2: Lwa crRNA colony 1; lane 3: Lwa crRNA colony 2; lane 4: Lwa crRNA colony 3; lane 5: Lbu crRNA colony 1; lane 6: Lbu crRNA colony 2; lane 7: Lbu crRNA colony 3; lanes 8-13: empty.
Figure 4. 1% agarose of restriction digests using PstI and EcoRI from Escherichia coli BL21(DE3) cells containing plasmids for RFP and either Lbu crRNA or Lwa crRNA. Left to right: lane 1: 1 kb ladder; lane 2: Lwa crRNA colony 1; lane 3: Lwa crRNA colony 2; lane 4: Lwa crRNA colony 3; lane 5: Lbu crRNA colony 1; lane 6: Lbu crRNA colony 2; lane 7: Lbu crRNA colony 3; lanes 8-13: empty.
As an alternative experiment, our team grew cells that expressed the Lbu and Lwa Cas13a protein overnight. We also grew cells that expressed dual plasmids; GFP and crRNA Lwa; GFP and crRNA Lbu; RFP and crRNA Lwa; and RFP and crRNA Lbu. We then lysed the cells that expressed the Cas13a proteins using a French Press and clarified the lysate via centrifugation. Following this, our team pipetted in a 1:1 ratio of clarified cell lysate: fluorescent protein and crRNA into a 96 well plate. This allowed us to observe if there would be an effect from the CRISPR Cas13a system on the fluorescent proteins. We observed that in our optical density data, both dual plasmid systems for GFP and RFP had stunted growth in comparison to only E. coli cells expressing GFP or RFP or no plasmid (Figure 5C). Adding the lysate may have caused the death of the culture. We neglected to include replicates of the dual plasmid system without adding lysate to observe how that grew. This would be beneficial for any future experiments. Alternatively, there may have been some effect of the protein in the lysate on the GFP fluorescence (Figure 5B). However, we are unsure of the specificity due to the potential of the RFP not folding correctly in vivo as demonstrated by the substantial standard deviation seen in our replicates (Figure 5A).
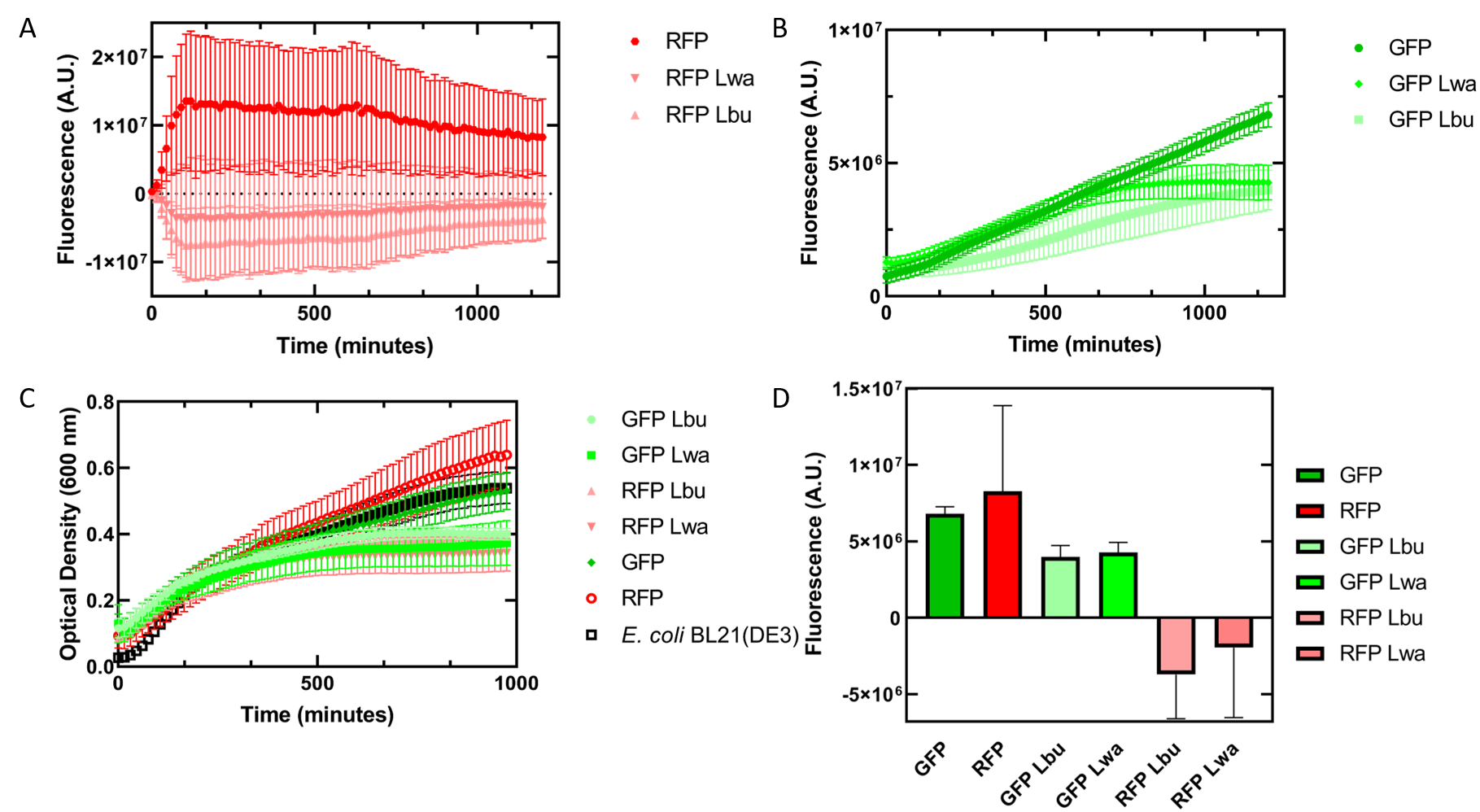 Figure 5 In vivo fluorescence assay of E. coli BL21(DE3) cells containing fluorescent protein and crRNA plasmids and E. coli Rosetta(DE3) cell lysate of overexpressed Cas13a proteins. This assay was conducted with 3 biological replicates and 3 technical replicates. (A) Fluorescence of E. coli cells containing only an RFP expressing plasmid, or dual plasmid expression of RFP and crRNAs from Lwa and Lbu that target GFP and the respective cell lysate containing the appropriate Cas13a. RFP excitation was at 558 nm and emission at 583 nm. (B) Fluorescence of E. coli cells containing only a GFP expressing plasmid, or dual plasmid expression of GFP and crRNAs from Lwa and Lbu that target GFP and the respective cell lysate containing the appropriate Cas13a. GFP excitation was at 475 nm and emission at 508 nm. (C) Optical density of E. coli cells expressing GFP, RFP, dual plasmid systems mentioned previously, or only E. coli BL21(DE3) cells with absorbance measured at 600 nm. (D) Relative fluorescence at maximum excitation at 81 minutes. GFP excitation was at 475 nm and emission at 508 nm and RFP excitation was at 558 nm and emission at 583 nm.
Figure 5 In vivo fluorescence assay of E. coli BL21(DE3) cells containing fluorescent protein and crRNA plasmids and E. coli Rosetta(DE3) cell lysate of overexpressed Cas13a proteins. This assay was conducted with 3 biological replicates and 3 technical replicates. (A) Fluorescence of E. coli cells containing only an RFP expressing plasmid, or dual plasmid expression of RFP and crRNAs from Lwa and Lbu that target GFP and the respective cell lysate containing the appropriate Cas13a. RFP excitation was at 558 nm and emission at 583 nm. (B) Fluorescence of E. coli cells containing only a GFP expressing plasmid, or dual plasmid expression of GFP and crRNAs from Lwa and Lbu that target GFP and the respective cell lysate containing the appropriate Cas13a. GFP excitation was at 475 nm and emission at 508 nm. (C) Optical density of E. coli cells expressing GFP, RFP, dual plasmid systems mentioned previously, or only E. coli BL21(DE3) cells with absorbance measured at 600 nm. (D) Relative fluorescence at maximum excitation at 81 minutes. GFP excitation was at 475 nm and emission at 508 nm and RFP excitation was at 558 nm and emission at 583 nm.
Applications of BBa_E1010
iGEM14_Carnegie_Mellon. We characterized a set of fluorescent proteins consisting of BFP. GFP, YFP, OFP, and RFP. We calculated the signal-to-noise ratio of all the proteins in two different cell lines (MACH and Top10). RFP had a high signal-to-noise ratio in both cell lines. RFP was measured at (ex/em = 584nm/607nm).


User Reviews
UNIQa29a4fa98bde2315-partinfo-00000000-QINU
|
•••••
Hong Kong-CUHK iGEM 2017 |
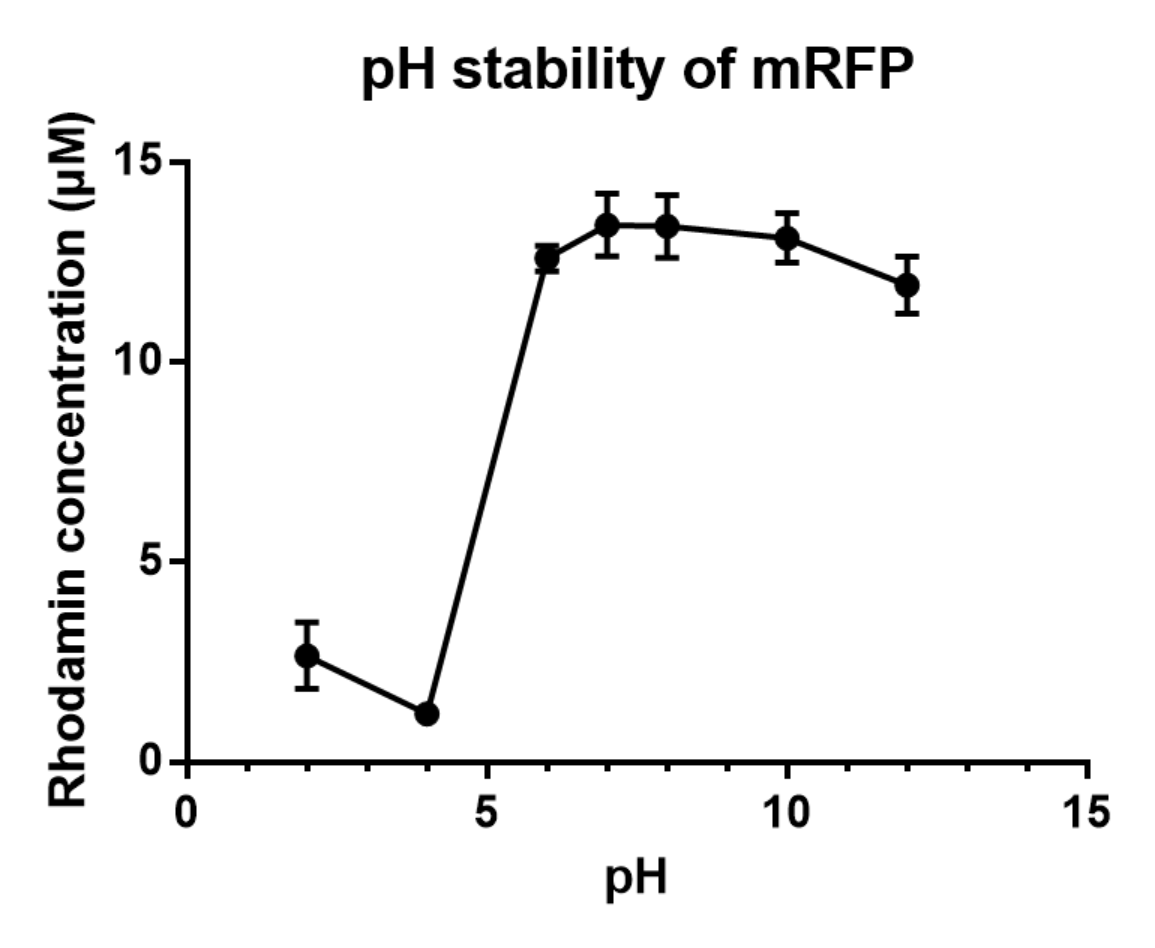
Charaterization of mRFP pH stabillity We grew C41 bacteria with parts BBa_J61002 in 2XYT for 24 hours. Purifying the mRFP by Ion Exchange Chromatography and Hydrophobic Interaction Chromatography, we measured the fluoresece (ex ,em ) of purified mRFP. which is diluted to 10µg/100µl (total 200µl) in triplicates, into different buffers (ranges from pH2 to pH12). The result shows that the stability drops dramatically in pH condition below 6 and relatively stable in pH 6-10.
|
|
•••••
Immudzen |
As part of the 2013 CU Boulder project we worked on separating RFP from AmilCP and during that process we ran into a problem that the fluorescence of RFP is too close to the absorption of AmilCP to tell them apart. What we ended up doing was measuring the spectrum of RFP from 400nm to 600nm. What we found is that RFP has a secondary absorbance peak at 502nm which is well clear of AmilCP. Under the devices we had access to this secondary peak also remained in the linear region of our device over a much wider concentration range. We also found that when running on an agarose gel RFP will run down on the gel while AmilCP runs up on the gel. |
|
•••••
HIT-Harbin |
1)Measuring absorbance of RFP
We grew bacteria without device(BBa_J04450) and bacteria with parts BBa_J04450 in same volume until stationary phase. Taking bacteria without device as background, we measured the absorbance of bacteria with our device (the max absorption peak is 504nm).But absorbance in 504nm is higher than 1,which present a bad linear relation between absorbance and concentraton. RFP has absorption in 450nm,and absorbance is between 0.1 and 1(better linear relation).Occasionally, we find a RFP standard curve under 450nm on the web.Before the mensuration, we diluted the two groups according to table1. We took the mean of two measures as the useful data. " Fig.1 RFP absorbance varying with wave length Table 1 Dilution of Two groups of bacteria " Fig 2. The relationship between RFP concentration and absorbance(OD450) 2)The actual relationship between RFP concentration and absorbance " Fig 3. RFP standard curve obtain from the web absolute concentration, and finally get the relationship between IPTG concentration and RFP concentration. Compared to crushing cells to separate RFP, our method is simpler and easy topractice. Moreover, our relative concentration curve is credible. If the standard curve is reliable, our calculated result of RFP will be precise. |
|
•••••
Carnegie_Mellon 2013 |
Characterization of the Photostability of mRFP1 XL10 Ultracompetent cells were transformed with BBa_E1010 cloned with BBa_B0034 as the RBS and BBa_R0010 as the wild-type lac promoter and induced overnight with IPTG.The overnight was bleached for 180 minutes with HBO100 (100W Mercury-arc lamp). Fluorescence data was taken using a Tecan Safire II with the parameters shown in Table 3. Fluorescence values are shown in Table 4.
|
|
••••
KAIST_iGEM_2012 |
 Figure 1. E.coli strain MG1655 expressing BBa_E1010 under control of BBa_K907005 after overnight culture. 3mL culture with M9 media in 14ml round bottom tube(left), and centrifuged cells in eppendorf tube(right). The expression of BBa_1010 is clearly observed with naked eye after overnight culture.
|
|
•••
DTU_igem_2010 |
Characterization of RFP BBa_E1010 Method Results
|
|
Antiquity |
This review comes from the old result system and indicates that this part did not work in some test. |
|
Nkessler |
We successfully used this part for a read out system, e.g. in BBa_K389016. Additionally we compared it with a luciferase: BBa_K389004. |


 "
"
 "
"
"
" "
"
 "
"