Difference between revisions of "Part:BBa K2455002"
(→SDS-PAGE) |
(→Purification) |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K2455002 short</partinfo> | <partinfo>BBa_K2455002 short</partinfo> | ||
| − | This is an improved version of the Super Yellow Fluorescent Protein 2 ([[Part:BBa_K864100|BBa_K864100]]) carrying an N-terminal nona- | + | This is an improved version of the Super Yellow Fluorescent Protein 2 ([[Part:BBa_K864100|BBa_K864100]]) carrying an N-terminal nona-arginine tag, and a C-terminal hexa-histidine tag. The R9-tag is a Cell-Penetrating Peptide giving it's associated protein the ability to pass through plasma membranes. |
For our study we demonstrated that this improved version of [[Part:BBa_K864100|SYFP2]] had fluorescence of similar levels to the original protein, and was able to stain both <i>Escherichia coli</i> and <i>Pseudomonas putida</i> cells, presumably through internalisation of the protein. | For our study we demonstrated that this improved version of [[Part:BBa_K864100|SYFP2]] had fluorescence of similar levels to the original protein, and was able to stain both <i>Escherichia coli</i> and <i>Pseudomonas putida</i> cells, presumably through internalisation of the protein. | ||
| Line 23: | Line 23: | ||
<span class='h3bb'><font size="5"><b>Usage and Biology</b></font></span> | <span class='h3bb'><font size="5"><b>Usage and Biology</b></font></span> | ||
===SYFP2=== | ===SYFP2=== | ||
| − | [[Part:BBa_K864100|SYFP2]] is a monomeric GFP-based protein | + | [[Part:BBa_K864100|SYFP2]] is a monomeric GFP-based protein emitting a bright yellow fluorescence. It has an excitation peak at 515 nm, an emission peak at 527 nm and a relative brightness compared to EYFP2(Q96K) of 12.0 in <i>E. coli</i>. |
This version of [[Part:BBa_K864100|SYFP2]] has an N-terminal R9-tag and a C-terminal H6-tag. | This version of [[Part:BBa_K864100|SYFP2]] has an N-terminal R9-tag and a C-terminal H6-tag. | ||
| Line 32: | Line 32: | ||
===Biobrick Creation=== | ===Biobrick Creation=== | ||
| − | The [[part:BBa_K2455002| | + | The [[part:BBa_K2455002|CPP-SYFP2]] biobrick was constructed by amplifying [[Part:BBa_K864100|SYFP2]] using primers with overhangs designed for insertion into the USER cassette. The PCR product was then purified and ligated into the vector, before being restriction digested and sequenced. |
==Purification and SDS-PAGE== | ==Purification and SDS-PAGE== | ||
| − | The [[Part:BBa_K864100|SYFP2]] and [[Part:BBa_K2455002| | + | The [[Part:BBa_K864100|SYFP2]] and [[Part:BBa_K2455002|CPP-SYFP2]] proteins were expressed and purified in order to test for internalisation of the proteins in bacteria. All expression were done in BL21 <i>Escherichia coli</i> under control of the T7 promoter. Protein expression was induced with an IPTG concentration of 1.0 mM as optimal expression levels were found at this concentration. |
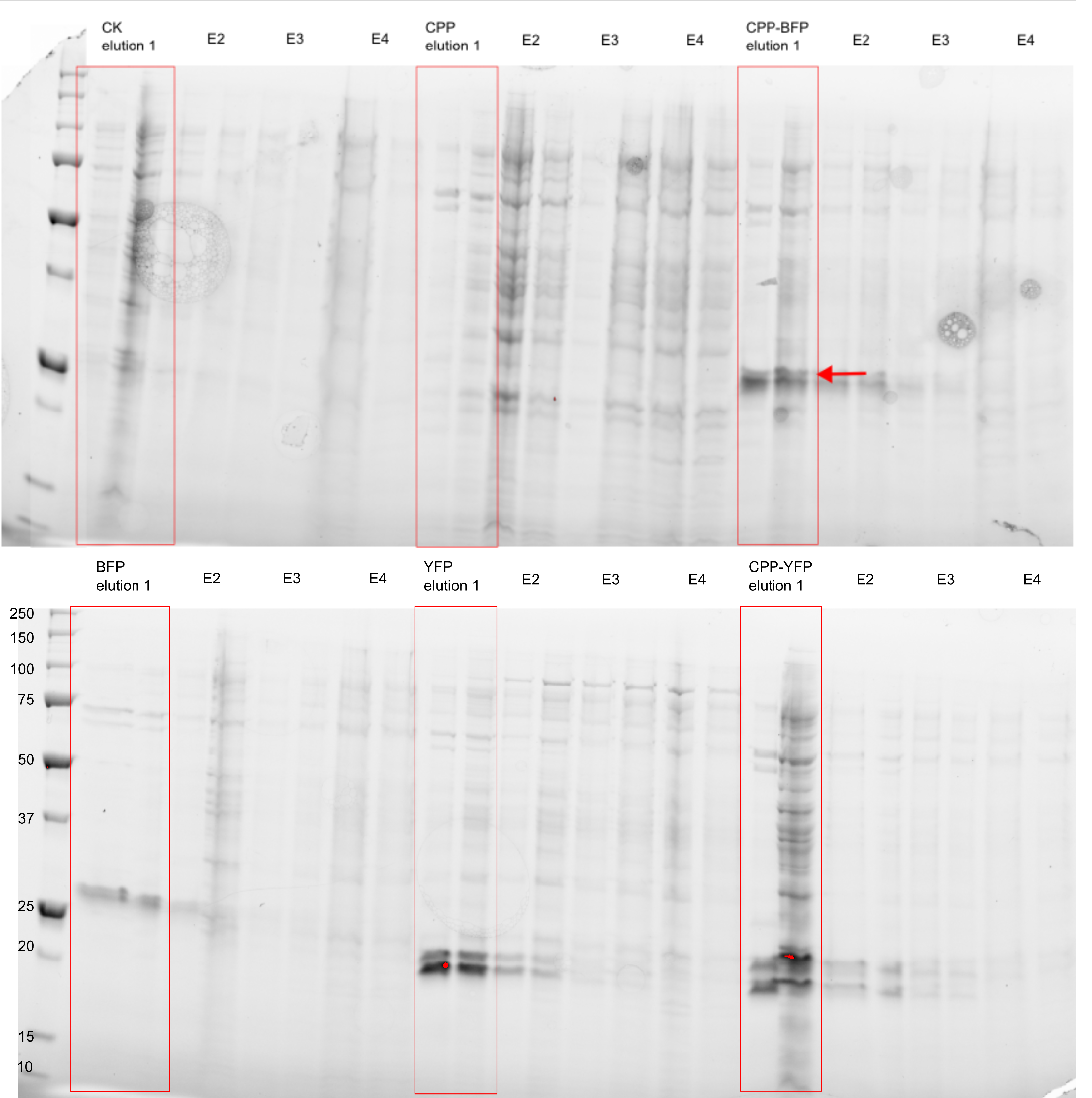
[[Image: My_western_image.png|300px|thumb|right|'''Figure 1: Purification of fluorescent proteins.''' Expressed proteins were purified using an Imidazole gradient; E1: 250 mM, E2: 500 mM, E3: 1.0 M, E4: 2.0 M]] | [[Image: My_western_image.png|300px|thumb|right|'''Figure 1: Purification of fluorescent proteins.''' Expressed proteins were purified using an Imidazole gradient; E1: 250 mM, E2: 500 mM, E3: 1.0 M, E4: 2.0 M]] | ||
===Purification=== | ===Purification=== | ||
| − | Cells were harvested, re-suspended in | + | Cells were harvested, re-suspended in lysis buffer (25 mM Imidazole, 0.5 M NaCl, 20 mM Tris-HCl (pH 7.9), and 1.0 mM PMSF), and lysed by sonication. The cell debris was removed by centrifugation at low speed, and the supernatant was transferred to a new falcon tube and mixed with 100 µL [https://www.qiagen.com/us/shop/sample-technologies/protein/expression-purification-detection/ni-nta-agarose/#orderinginformation Ni-NTA Agarose]. The mixture was incubated for one hour at 4 degrees Celcius on a turning table, where after the beads were spun down and the supernatant removed. A series of elution buffers (0.5 M NaCl, 20 mM Tris-HCl; pH 7.9) with increasing Imidazole concentration were used to elute the bound proteins. The elution buffers were added consecutively 100 µl each time followed by centrifuging the sample at low speed, and removing 100 µL eluate. |
<b>NOTE:</b> Samples were kept on ice at all times and centrifugation took place at 4 degrees Celsius. | <b>NOTE:</b> Samples were kept on ice at all times and centrifugation took place at 4 degrees Celsius. | ||
===SDS-PAGE=== | ===SDS-PAGE=== | ||
| − | The purity of the samples was analysed by subjecting them to an SDS-PAGE using a [http://www.bio-rad.com/en-cn/product/criterion-precast-gels/criterion-tgx-stain-free-precast-gels pre-cast stain free gel]. In addition to [[Part:BBa_K864100|SYFP2]] and [[Part:BBa_K2455002| | + | The purity of the samples was analysed by subjecting them to an SDS-PAGE using a [http://www.bio-rad.com/en-cn/product/criterion-precast-gels/criterion-tgx-stain-free-precast-gels pre-cast stain free gel]. In addition to [[Part:BBa_K864100|SYFP2]] and [[Part:BBa_K2455002|CPP-SYFP2]], two versions of the [[Part:BBa_K592100|mTag BFP]] biobrick were also purified. The Imidazole concentration of the purification ranged from 250 mM to 2.0 M, as can be seen on Figure 1 proteins of the expected size are eluted at Imidazole concentrations of 250 mM for all constructs (BFP & YFP 28kDa, CPP-BFP & CPP-YFP 30kDa). |
==Imaging== | ==Imaging== | ||
| − | In addition to [[Part:BBa_K2455002| | + | In addition to [[Part:BBa_K2455002|CPP-SYFP2]] the [[Part:BBa_K2455005|CPP-mTag BFP]] biobrick was also analysed. All fluorescent imaging was done at 100x magnification using the following setup: |
<b>Yellow fluorescence</b>: | <b>Yellow fluorescence</b>: | ||
| Line 72: | Line 72: | ||
===Fluorescent Imaging of Expressing Cells=== | ===Fluorescent Imaging of Expressing Cells=== | ||
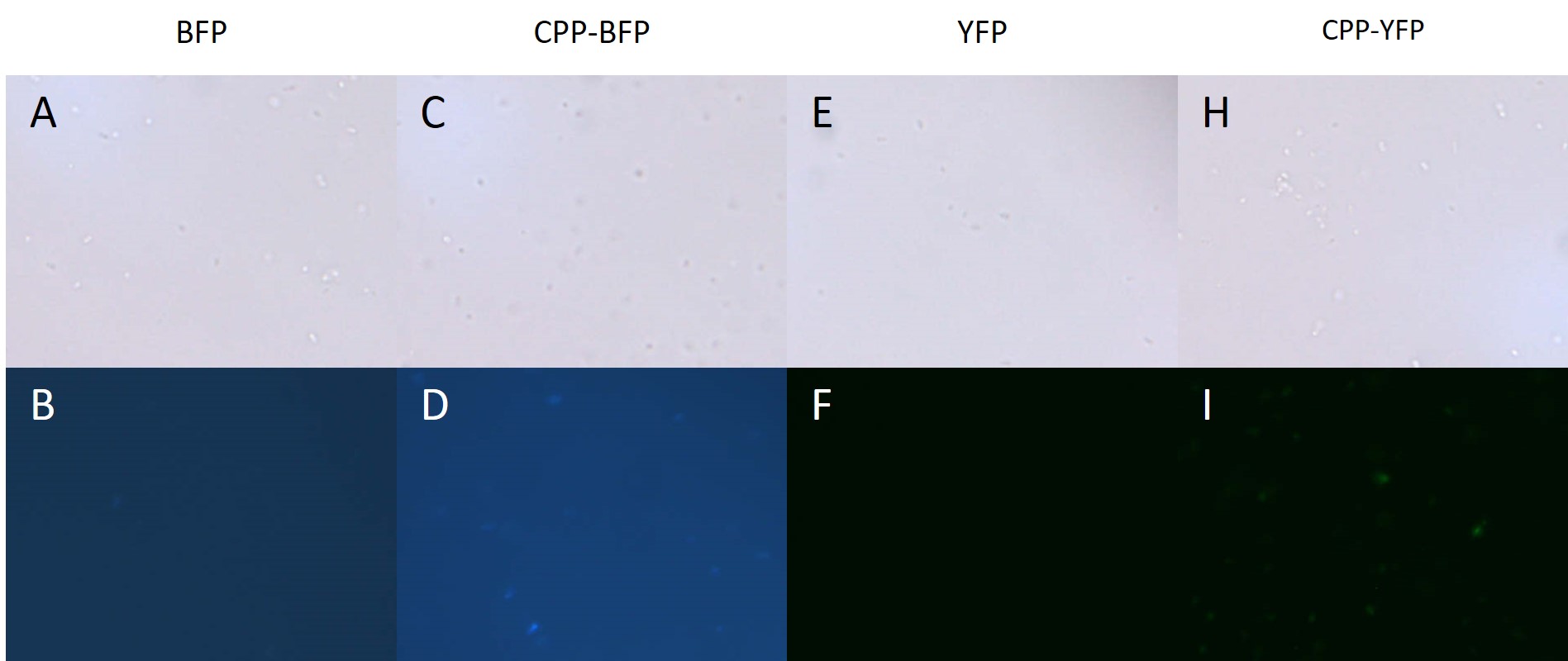
| − | BL21 <i>E. coli</i> expressing either [[Part:BBa_K864100|SYFP2]], [[Part:BBa_K592100|mTag BFP]], [[Part:BBa_K2455002| | + | BL21 <i>E. coli</i> expressing either [[Part:BBa_K864100|SYFP2]], [[Part:BBa_K592100|mTag BFP]], [[Part:BBa_K2455002|CPP-SYFP2]], and [[Part:BBa_K2455005|CPP-mTag BFP]] were analysed on a fluorescent microscope at 100x magnification in order to see if the fusion proteins were capable of emitting fluorescent light as their parental counterparts. As can be see on Figure 2 the intensity of the individual cells were as bright with the CPP tag as without. |
| − | [[Image: E.coli_export_large.png|600px|thumb|center|'''Figure 2: BL21 <i>E. coli</i> cells expressing either fluorescent proteins.''' A-F) As expected no fluorescence is seen in cells carrying either an empty vector or a vector with an empty [[Part:BBa_K2455003|Cell-Penetrating USER Cassette]]. G+I) Fluorescence of at least equal intensity is seen in cells expressing [[Part:BBa_K2455005| | + | [[Image: E.coli_export_large.png|600px|thumb|center|'''Figure 2: BL21 <i>E. coli</i> cells expressing either fluorescent proteins.''' A-F) As expected no fluorescence is seen in cells carrying either an empty vector or a vector with an empty [[Part:BBa_K2455003|Cell-Penetrating USER Cassette]]. G+I) Fluorescence of at least equal intensity is seen in cells expressing [[Part:BBa_K2455005|CPP-mTag BFP]] as to those expressing [[Part:BBa_K592100|mTag BFP]]. H+J) Equal fluorescence is seen for cells expressing [[Part:BBa_K864100|SYFP2]] and [[Part:BBa_K2455002|CPP-SYFP2]]]] |
===Cell-Penetrating Capabilities of Purified Proteins=== | ===Cell-Penetrating Capabilities of Purified Proteins=== | ||
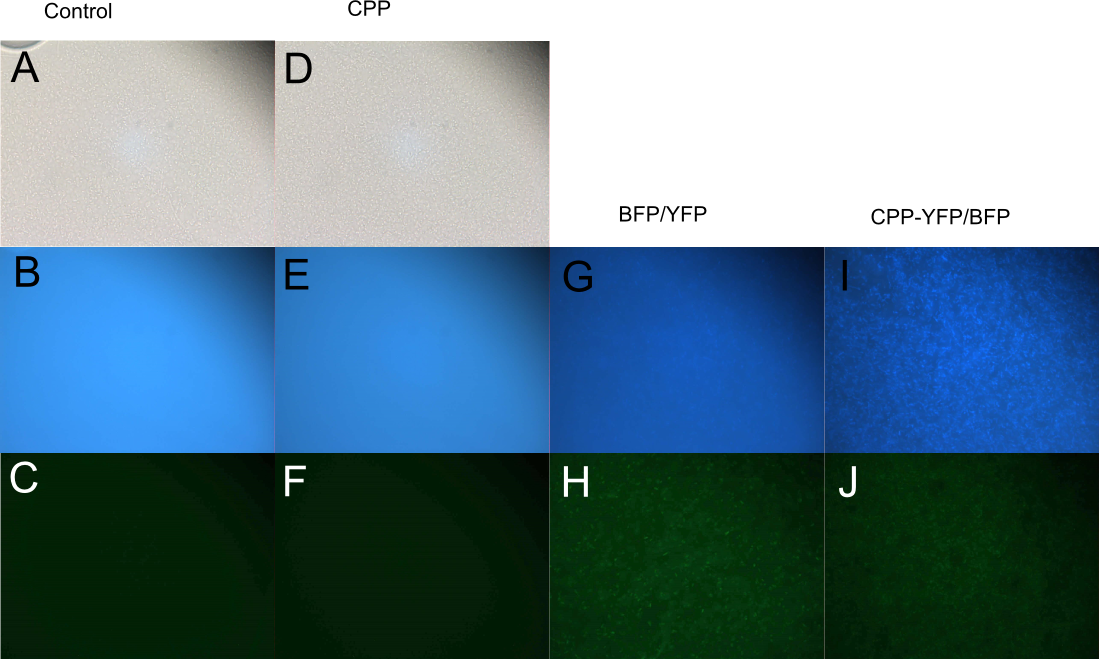
| − | In order to investigate the capabilities of the expressed proteins to enter <i>E. coli</i> cells, 10 µL of <i>E. coli</i> MG1655 overnight culture was mixed with 25 µL purified protein from either Control (empty vector), [[Part:BBa_K864100|SYFP2]], [[Part:BBa_K592100|mTag BFP]], [[Part:BBa_K2455002|CPP]], [[Part:BBa_K2455002| | + | In order to investigate the capabilities of the expressed proteins to enter <i>E. coli</i> cells, 10 µL of <i>E. coli</i> MG1655 overnight culture was mixed with 25 µL purified protein from either Control (empty vector), [[Part:BBa_K864100|SYFP2]], [[Part:BBa_K592100|mTag BFP]], [[Part:BBa_K2455002|CPP]], [[Part:BBa_K2455002|CPP-SYFP2]], or [[Part:BBa_K2455005|CPP-mTag BFP]] and incubated at room temperature for 10 min. Subsequently the cells were pelleted and the supernatant was removed, followed by 2 x wash in 500 mL MQ H<sub>2</sub>O. Finally the cells were re-suspended in 10 µL MQ H<sub>2</sub>O before being imaged on the fluorescent microscope. As can be seen on Figure 3, while neither [[Part:BBa_K864100|SYFP2]] or [[Part:BBa_K592100|mTag BFP]] alone were capable of staining the cells, both [[Part:BBa_K2455002|CPP-SYFP2]] and [[Part:BBa_K2455005|CPP-mTag BFP]] were able too, thus indicating an ability of the [[Part:BBa_K2455003|Cell-Penetrating USER Cassette]] to facilitate internalization of their protein cargo. |
| − | [[Image: Slack-imgs.png|700px|thumb|center|'''Figure 3: Fluorescence of <i>E. coli</i> cells treated with fluorescent protein.''' Cells were treated with either [[Part:BBa_K592100|mTag BFP]] (A-B), [[Part:BBa_K2455005| | + | [[Image: Slack-imgs.png|700px|thumb|center|'''Figure 3: Fluorescence of <i>E. coli</i> cells treated with fluorescent protein.''' Cells were treated with either [[Part:BBa_K592100|mTag BFP]] (A-B), [[Part:BBa_K2455005|CPP-mTag BFP]] (C-D), [[Part:BBa_K864100|SYFP2]] (E-F), or [[Part:BBa_K2455002|CPP-SYFP2]] (G-H). Cells treated with CPP-tagged protein emitted noticeably more fluorescence.]] |
| − | In addition to <i>E. coli</i>, both <i>Pseudomonas putida</i> and <i>Bacillus subtilis</i> were similarly treated with [[Part:BBa_K864100|SYFP2]], [[Part:BBa_K592100|mTag BFP]], [[Part:BBa_K2455002| | + | In addition to <i>E. coli</i>, both <i>Pseudomonas putida</i> and <i>Bacillus subtilis</i> were similarly treated with [[Part:BBa_K864100|SYFP2]], [[Part:BBa_K592100|mTag BFP]], [[Part:BBa_K2455002|CPP-SYFP2]], or [[Part:BBa_K2455005|CPP-mTag BFP]] following the same protocol as for <i>E. coli</i> (see above). While <i>B. subtilis</i> showed no signs of protein uptake (data not shown), <i>P. putida</i> showed similar signs to protein uptake as did <i>E. coli</i> (see Fig. 4). |
| − | [[Image: Pseudomonas PP.jpg|700px|thumb|center|'''Figure 4: Fluorescence of <i>P. putida</i> cells treated with fluorescent protein.''' Cells were treated with either [[Part:BBa_K592100|mTag BFP]] (A-B), [[Part:BBa_K2455005| | + | [[Image: Pseudomonas PP.jpg|700px|thumb|center|'''Figure 4: Fluorescence of <i>P. putida</i> cells treated with fluorescent protein.''' Cells were treated with either [[Part:BBa_K592100|mTag BFP]] (A-B), [[Part:BBa_K2455005|CPP-mTag BFP]] (C-D), [[Part:BBa_K864100|SYFP2]] (E-F), or [[Part:BBa_K2455002|CPP-SYFP2]] (G-H). Cells treated with CPP-tagged protein emitted noticeably more fluorescence.]] |
Latest revision as of 01:20, 2 November 2017
CPP-SYFP2
This is an improved version of the Super Yellow Fluorescent Protein 2 (BBa_K864100) carrying an N-terminal nona-arginine tag, and a C-terminal hexa-histidine tag. The R9-tag is a Cell-Penetrating Peptide giving it's associated protein the ability to pass through plasma membranes.
For our study we demonstrated that this improved version of SYFP2 had fluorescence of similar levels to the original protein, and was able to stain both Escherichia coli and Pseudomonas putida cells, presumably through internalisation of the protein.
Previous studies have shown R9 associated proteins to be able to enter a variety of cell types (Chang et al., 2005).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
SYFP2
SYFP2 is a monomeric GFP-based protein emitting a bright yellow fluorescence. It has an excitation peak at 515 nm, an emission peak at 527 nm and a relative brightness compared to EYFP2(Q96K) of 12.0 in E. coli.
This version of SYFP2 has an N-terminal R9-tag and a C-terminal H6-tag.
Cell-Penetrating Peptides
Cell-Penetrating Peptides (CPPs) are small peptides, that are able to facilitate transport of a wide variety of cargoes across plasma membranes. CPPs are typically rich in basic residues and originate from viral domains such as the viral HIV tat domain (Eudes & Chugh, 2008). In recent years researchers have tried to make synthetic CPPs, with special interest in peptides constructed solely from arginine residues. The arginine rich sequences have been shown to trigger endocytosis in a wide range of cell types, including onion and potato cells (Chang et al., 2005).
Biobrick Creation
The CPP-SYFP2 biobrick was constructed by amplifying SYFP2 using primers with overhangs designed for insertion into the USER cassette. The PCR product was then purified and ligated into the vector, before being restriction digested and sequenced.
Purification and SDS-PAGE
The SYFP2 and CPP-SYFP2 proteins were expressed and purified in order to test for internalisation of the proteins in bacteria. All expression were done in BL21 Escherichia coli under control of the T7 promoter. Protein expression was induced with an IPTG concentration of 1.0 mM as optimal expression levels were found at this concentration.
Purification
Cells were harvested, re-suspended in lysis buffer (25 mM Imidazole, 0.5 M NaCl, 20 mM Tris-HCl (pH 7.9), and 1.0 mM PMSF), and lysed by sonication. The cell debris was removed by centrifugation at low speed, and the supernatant was transferred to a new falcon tube and mixed with 100 µL Ni-NTA Agarose. The mixture was incubated for one hour at 4 degrees Celcius on a turning table, where after the beads were spun down and the supernatant removed. A series of elution buffers (0.5 M NaCl, 20 mM Tris-HCl; pH 7.9) with increasing Imidazole concentration were used to elute the bound proteins. The elution buffers were added consecutively 100 µl each time followed by centrifuging the sample at low speed, and removing 100 µL eluate.
NOTE: Samples were kept on ice at all times and centrifugation took place at 4 degrees Celsius.
SDS-PAGE
The purity of the samples was analysed by subjecting them to an SDS-PAGE using a [http://www.bio-rad.com/en-cn/product/criterion-precast-gels/criterion-tgx-stain-free-precast-gels pre-cast stain free gel]. In addition to SYFP2 and CPP-SYFP2, two versions of the mTag BFP biobrick were also purified. The Imidazole concentration of the purification ranged from 250 mM to 2.0 M, as can be seen on Figure 1 proteins of the expected size are eluted at Imidazole concentrations of 250 mM for all constructs (BFP & YFP 28kDa, CPP-BFP & CPP-YFP 30kDa).
Imaging
In addition to CPP-SYFP2 the CPP-mTag BFP biobrick was also analysed. All fluorescent imaging was done at 100x magnification using the following setup:
Yellow fluorescence:
- Excitation time: 250 ms
- Excitation: 460 - 490 nm
- Dichroic Mirror: 505 nm
- Barrier Filter: 510 - 555 nm
Blue fluorescence:
- Excitation time: 100 ms
- Excitation: 330 - 385 nm
- Dichroic Mirror: 400 nm
- Barrier Filter: >420 nm
Fluorescent Imaging of Expressing Cells
BL21 E. coli expressing either SYFP2, mTag BFP, CPP-SYFP2, and CPP-mTag BFP were analysed on a fluorescent microscope at 100x magnification in order to see if the fusion proteins were capable of emitting fluorescent light as their parental counterparts. As can be see on Figure 2 the intensity of the individual cells were as bright with the CPP tag as without.
Cell-Penetrating Capabilities of Purified Proteins
In order to investigate the capabilities of the expressed proteins to enter E. coli cells, 10 µL of E. coli MG1655 overnight culture was mixed with 25 µL purified protein from either Control (empty vector), SYFP2, mTag BFP, CPP, CPP-SYFP2, or CPP-mTag BFP and incubated at room temperature for 10 min. Subsequently the cells were pelleted and the supernatant was removed, followed by 2 x wash in 500 mL MQ H2O. Finally the cells were re-suspended in 10 µL MQ H2O before being imaged on the fluorescent microscope. As can be seen on Figure 3, while neither SYFP2 or mTag BFP alone were capable of staining the cells, both CPP-SYFP2 and CPP-mTag BFP were able too, thus indicating an ability of the Cell-Penetrating USER Cassette to facilitate internalization of their protein cargo.
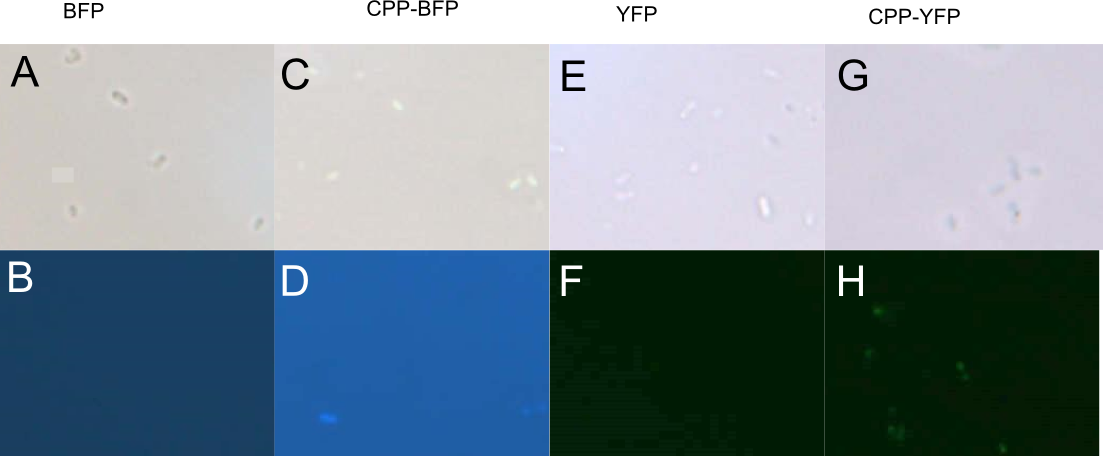
In addition to E. coli, both Pseudomonas putida and Bacillus subtilis were similarly treated with SYFP2, mTag BFP, CPP-SYFP2, or CPP-mTag BFP following the same protocol as for E. coli (see above). While B. subtilis showed no signs of protein uptake (data not shown), P. putida showed similar signs to protein uptake as did E. coli (see Fig. 4).