Part:BBa_K863207
tvel5 integrated in shuttle vector pECPP11JS (BBa_K863204) for integration in yeast
Shuttle vector for integration of tvel5 laccase from Trametes versicolor in yeast
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal XbaI site found at 5558
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 4853
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 3
Illegal BglII site found at 1931
Illegal BglII site found at 2363
Illegal BglII site found at 3004
Illegal BamHI site found at 3361
Illegal XhoI site found at 1198
Illegal XhoI site found at 1963
Illegal XhoI site found at 2596 - 23INCOMPATIBLE WITH RFC[23]Illegal XbaI site found at 5558
- 25INCOMPATIBLE WITH RFC[25]Illegal XbaI site found at 5558
Illegal NgoMIV site found at 2449
Illegal NgoMIV site found at 2602
Illegal AgeI site found at 1330 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 5714
Illegal BsaI.rc site found at 1885
Illegal BsaI.rc site found at 3513
Illegal SapI.rc site found at 1395
TVEL5 integrated in shuttle vector
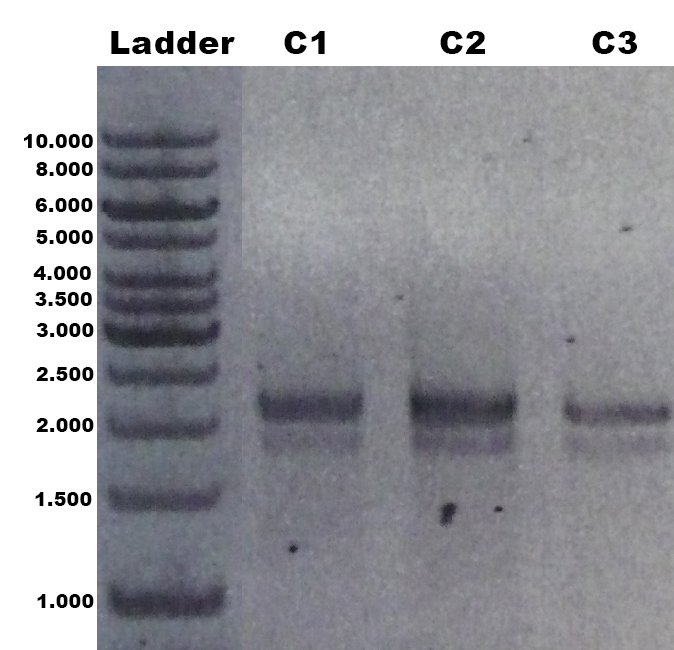
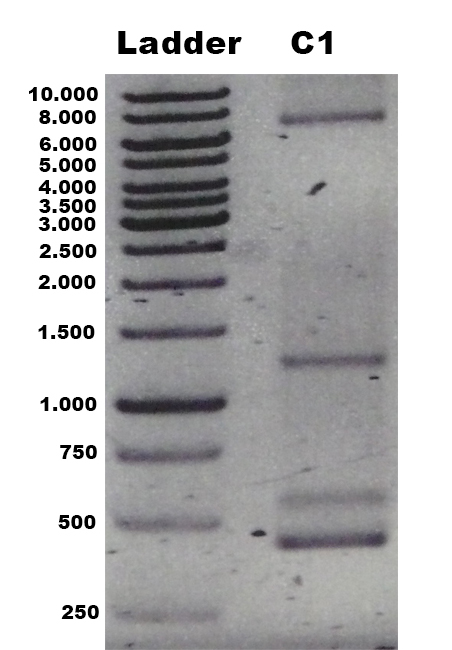
The laccase Trametes versicolor TVEL5 BBa_K863030 was cloned via the AarI restriction site into the shuttle vector (BBa_K863204). This construct (BBa_K863207) was linearized and integrated into the yeast genome of P. pastoris GS115. Positive clones with single cross overs were identified by PCR on genomic DNA with the primer 5AOX-Phenotype-FW (GACTGGTTCCAATTGACAAGC) and TT-Phenotype-RV (GCAAATGGCATTCTGACATCC) as shown in Figure 1 and 2. Positive clones with single cross over resulted in a 2.2 kb DNA band, the aox1 gene, and a 1.9 kb DNA band, the TVEL5 laccase DNA band. As control the shuttle vector (BBa_K863204) itself was used. The expected DNA size is 437 bp.
Cultivation and protein induction
The clones were cultivated in minimal medium and induced with 1% (v/v) methanol twice a day for 2 days.
Enzyme activity test
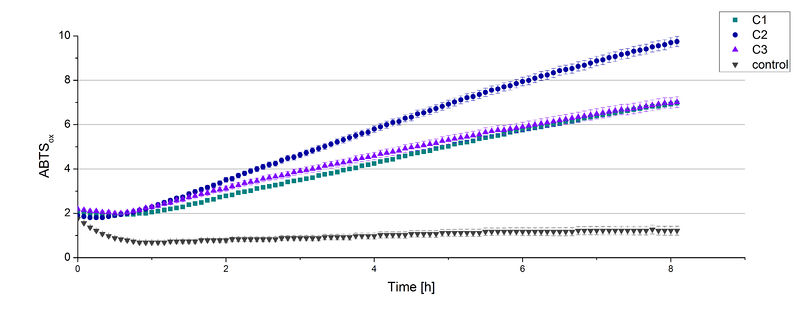
The supernatant of the culture was examined to active laccases by testing the enzyme activity. For this purpose the supernatant was re-buffered into H2O with HiTrap Desalting Columns and incubated with 0.4 mM CuCl2. After 2 hours of incubation time 140 µL of the re-buffered supernatant were applied for the activity assay with 40 µL Britton Robinson buffer (pH 5) and measured at 25 °C. These setting were chosen because of the experiences with the TVEL0 laccase. To detect activity of the TVEL5 laccase 9 mM ABTS were used. Figure 3 shows a distinct activity of each cultivation (C1, C2, C3) in comparison to the control, which was treated like the other samples. The control, which was also treated with CuCl2 also represented the negative control to determine the effect of CuCl2 oxidizing ABTS. In Figure 4 the same experimental setup was used in a total volume of 1 mL. The oxidation of ABTS by TVEL5 laccase is visible to the naked eye in C1, C2 and C3 in comparison to the control. C2 shows the highest potential in oxidizing ABTS in the activity measurements and also in the experiments with a higher volume. This may be due to a higher TVEL5 concentration in the supernatant of this cultivation.

| None |