Part:BBa_K3823012
hydrogen sulfide sensitive, three-gear adjustable kill switch
Design
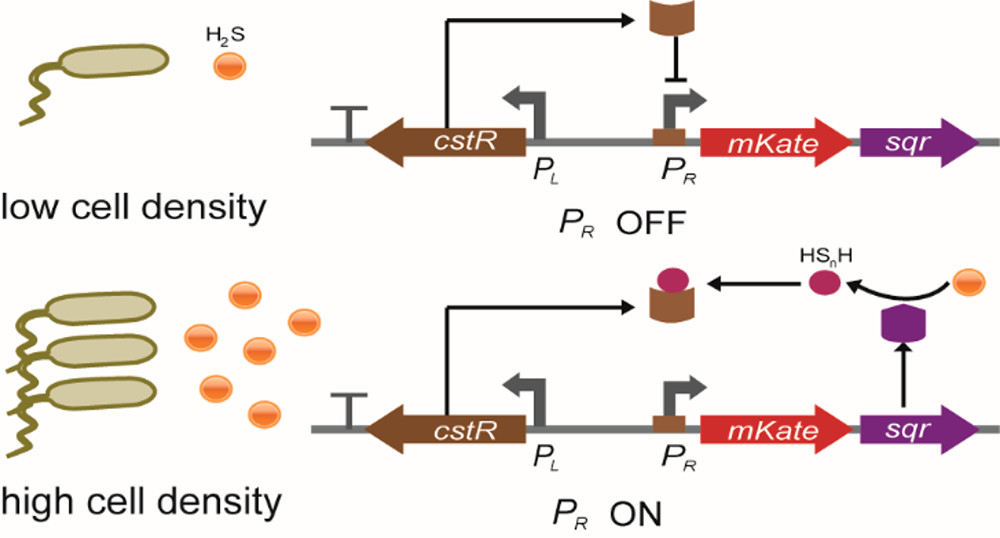
Biosafety has always been an important part in iGEM. To prevent our engineered bacteria from leaking into the environment, we designed a H2S sensitive three-gear kill switch to ensure this. H2S is an important factor in the working environment of our engineered bacteria, so we chose to use H2S concentration to control the on-off of the kill switch. By searching for the biosensors of various gas molecules, we found a HSnH-sensing biosensor (the product of SQR oxidized H2S happens to be HSnH) -- a gene regulator, CstR, which can sense HSnH and turn on the expression of the downstream genes (Figure 1.)[1].
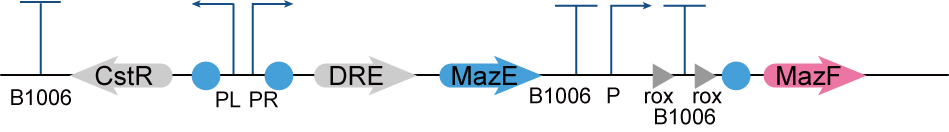
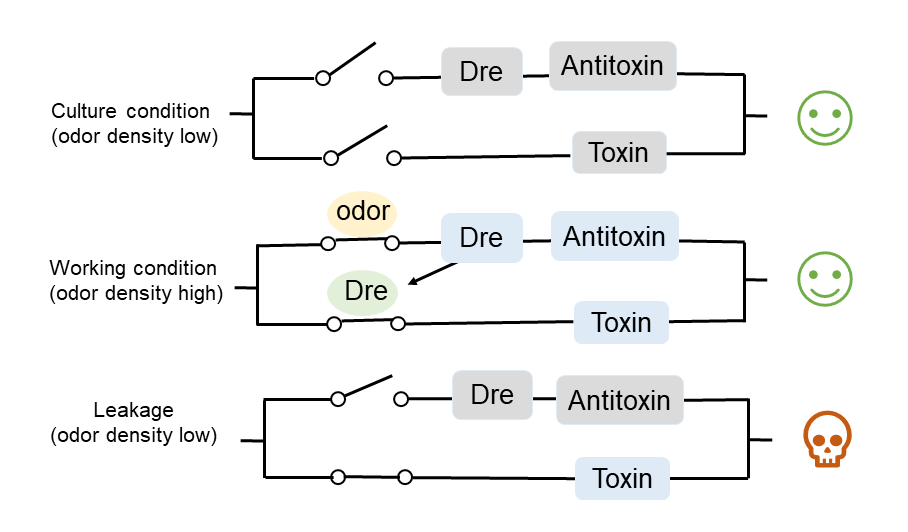
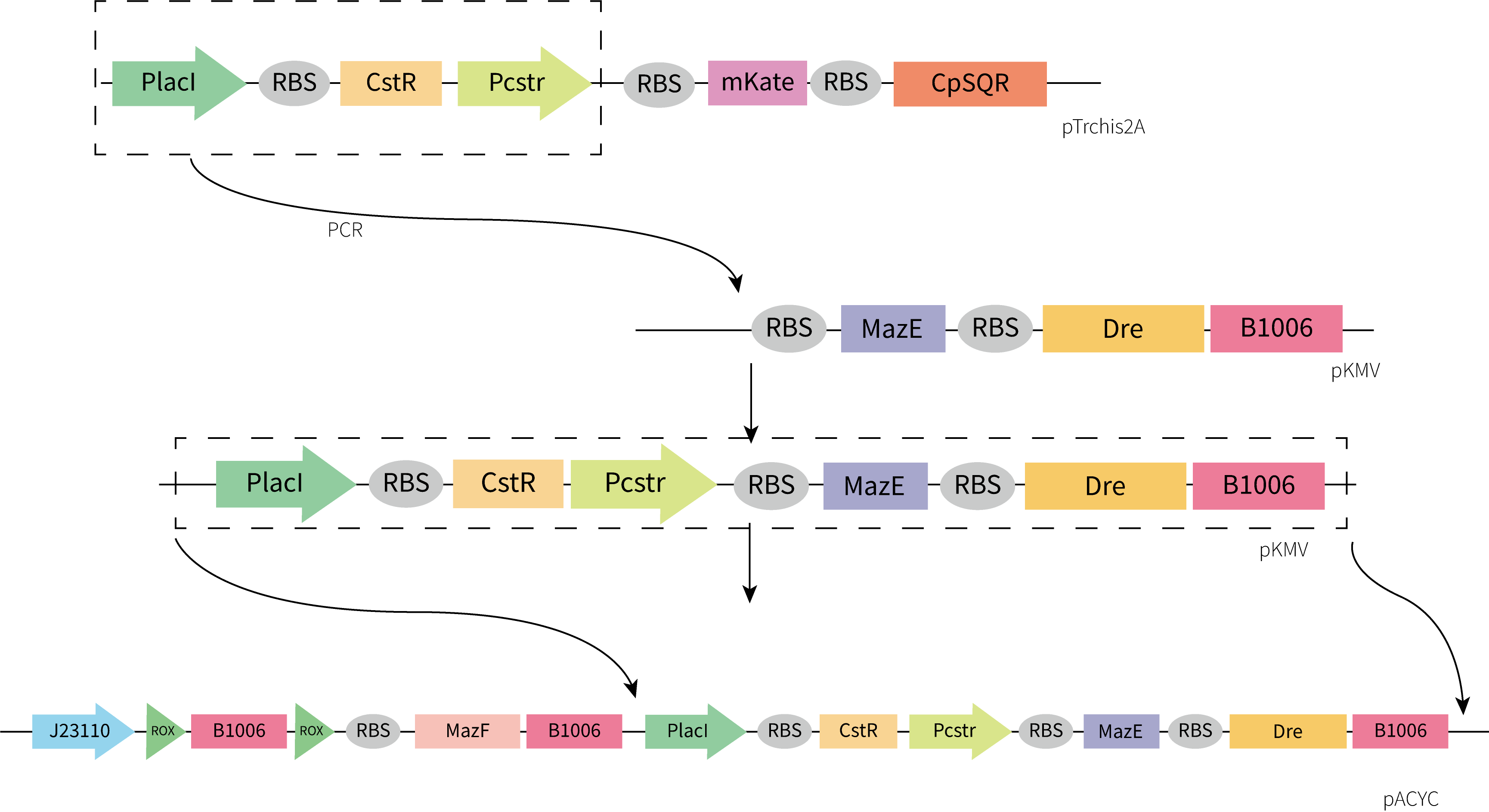
If we design a two-gear switch, namely, the switch is off when H2S concentration is high and on when H2S concentration is low, then we need to maintain high concentration of H2S in the culture environment, but this is obviously not realistic. Therefore, we designed a three-gear kill switch based on the Dre/rox recombinant enzyme system [2](referring to the work of the [http://2017.igem.org/Team:Edinburgh_UG Edinburgh UG in 2017] and the MazEF toxin-antitoxin system[3], which can achieve more flexible regulation of the survival of our engineered bacteria. (Figure 2.)
Under the culture condition, H2S concentration is not high enough to turn on any of the three pathways, so the bacteria will grow normally. When the concentration of H2S is high, H2S will remove the inhibition of CstR on the downstream gene expression by combining with it, then Dre will be expressed, and the terminator before MazF will be removed, resulting in the expression of MazF toxin. Meanwhile, due to the expression of the antitoxin MazE, the bacteria will not die. When working bacteria leak into the environment, H2S concentration in the environment will return to the normal (low) level, the inhibition of CstR will not be removed, and MazE will no longer be expressed. At this time, because MazF has been expressed, a large amount of MazF will kill the bacteria over time. (Figure 3.)
Experiments
Plasmid construction
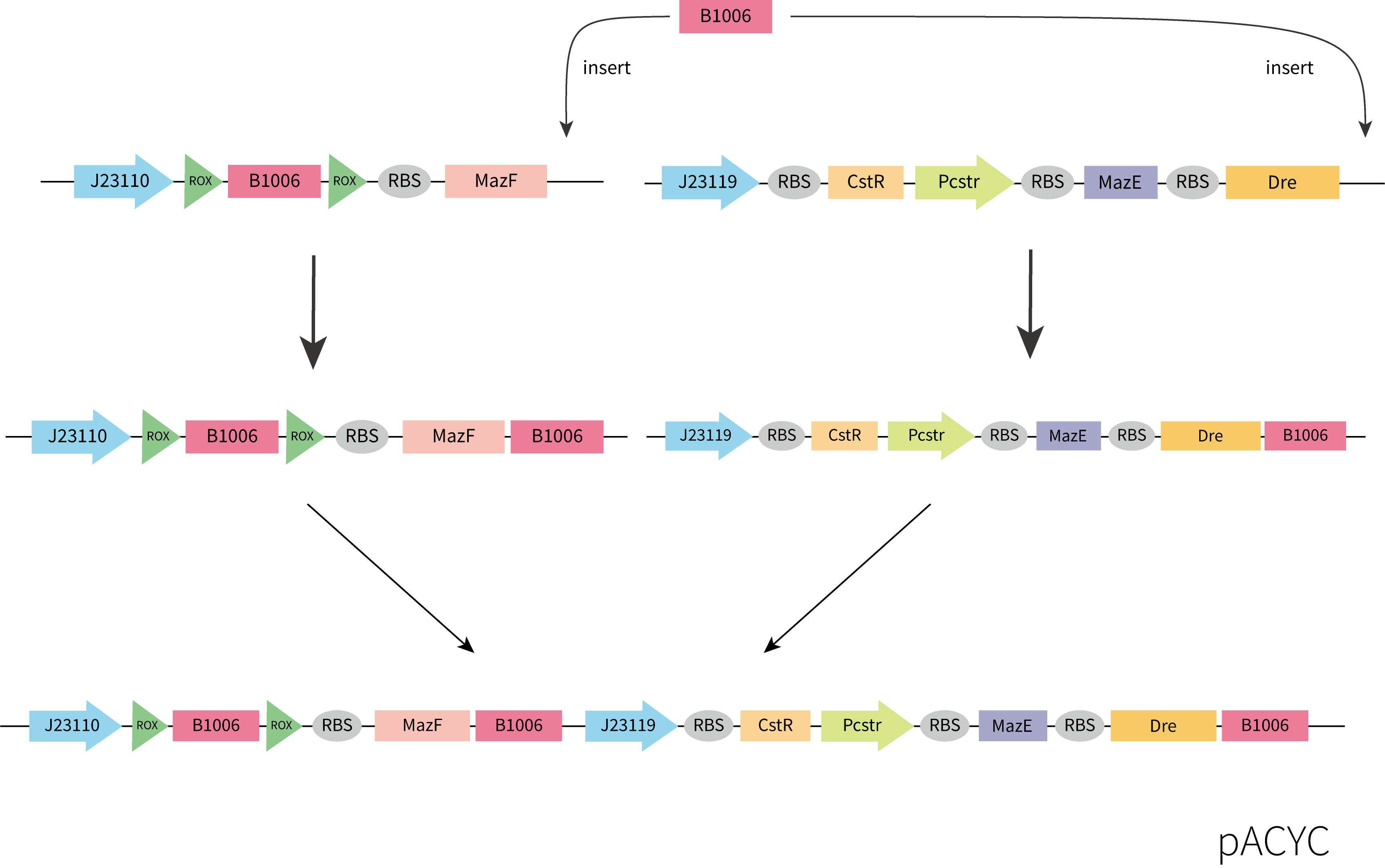
Considering the accessibility of the template and the time limitation, we adopted the strategy of assembling the functional components separately obtained by gene synthesis. Considering plasmid incompatibility, we chose pACYC as the vector for this part, which has a different replication system from pET-3a. Plasmids were constructed by restriction enzyme digestion and ligation and In-Fusion. The construction process we designed is shown below.
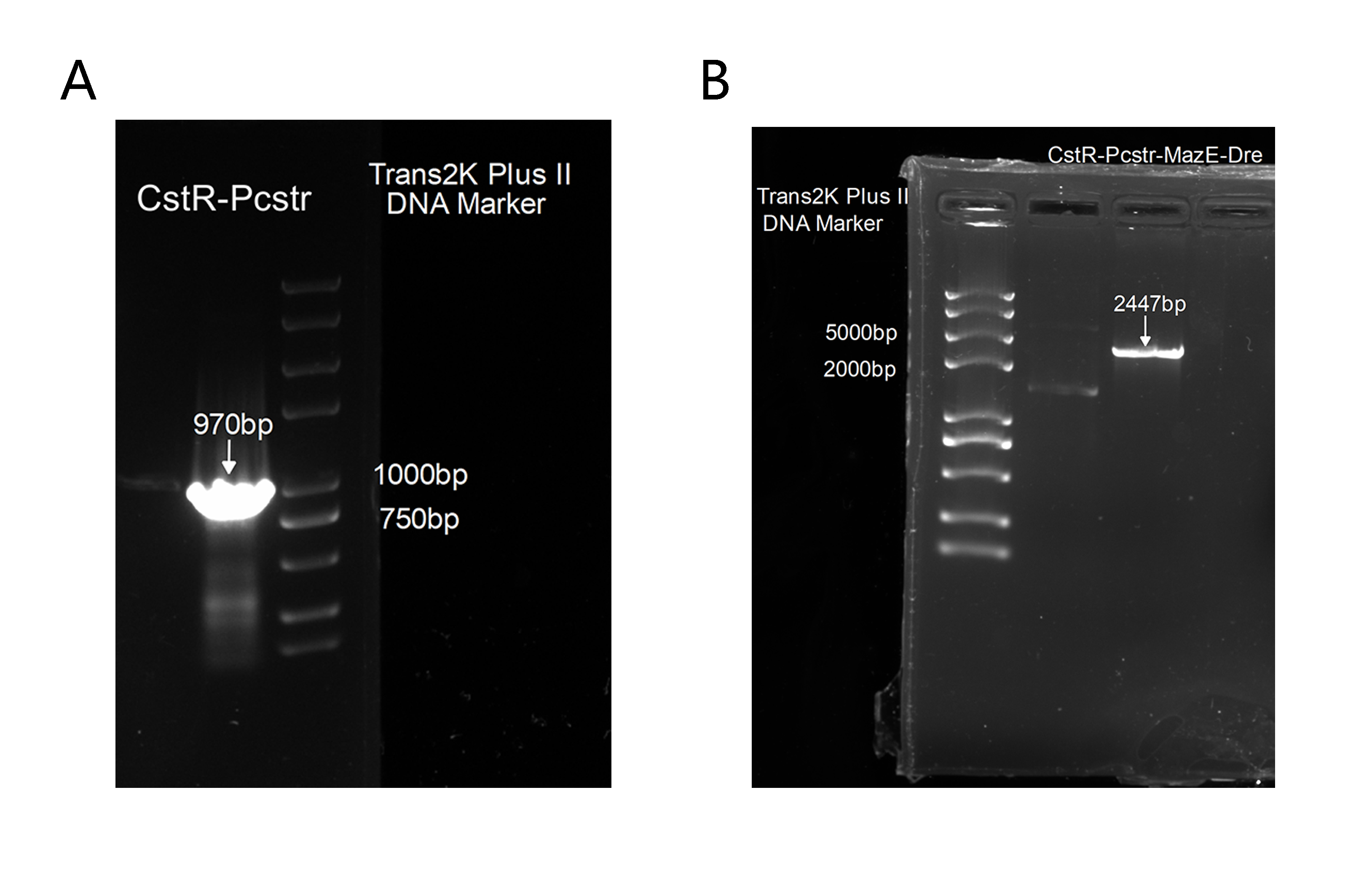
Unfortunately, the synthesis of J23119-CstR-Pcstr-MazE -Dre encountered difficulty so that we couldn't obtain the sequence of J23119-CstR-Pcstr. Considering this part was very necessary for our work, we contacted Professor Huaiwei Liu from Shandong University based on literature and fortunately got his help. Using the plasmid he donated as a template, we successfully amplified the CstR-Pcstr sequence(Figure 6A) and used it for our plasmid construction.
We successfully constructed the plasmid CstR-Pcstr-MazE -Dre and amplified CstR-Pcstr-MazE-Dre(BBa_K3823010)(Figure 6B).Then we cloned CstR-Pcstr-MazE-Dre to pACYC-rox-ter-rox-MazF.
However, according to the sequencing results, we found that Pcstr was missing(not in the PCR product), probably due to the complexity of each block. Meanwhile, we found the terminator between the two rox sites was partly moved after we cultured the final plasmid. We assumed that it could be the leakage of Pcstr that turned on the expression of Dre, which then cut off the terminator. This means we need to optimize our design. To optimize the design of our kill switch, we tried to find a proper combination of the three key promoters, PR(Pcstr), PL(PlacI), and J23110, by testing their strength respectively first.The results of this can be found in our another part pageBBa_K3823008 . From our results, we found that the intensity of Pcstr is significantly higher than J23110, and PlacI is very lower than J23110. This may explain why we encountered the leakage of Pcstr —— the inhibition of the CstR may be too low to block Pcstr while the intensity of Pcstr is in a high level. Although this is not consistent with the literature, the facts tell us that it is. Considering the time limitation and the workload of changing different promoters to test the best combination, we constructed a model to achieve this.
CstR with Pcstr Characterization
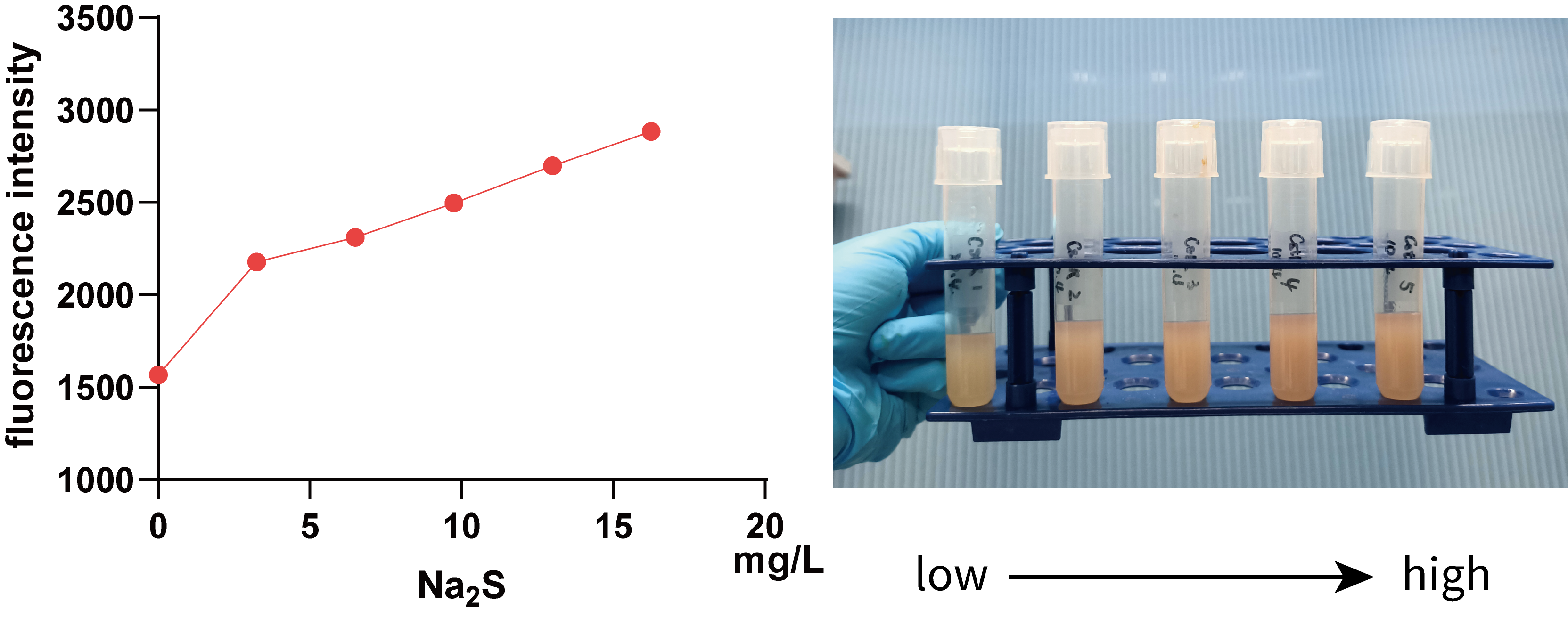
To ensure appropriate concentration range of S2- used for the characterization of pACYC-rox-ter-rox-MazF-CstR-Pcstr-MazE-Dre, we first characterized the function of CstR with Pcstr using pTrchis2A-CstR-Pcstr-mKate-CpSQR. The expression level regulated by Pcstr at different concentrations of S2- was shown by the fluorescence intensity of mKate(/OD600 nm). It can be seen that in a smaller concentration range, there is a positive correlation between fluorescence intensity and Na2S concentration, visible to naked eyes, which implies that we can use data within this concentration range to guide our characterization experiments.(The detailed results can be found in the BBa_K3823008).
But unfortunately, as mentioned above, we encountered difficulties in constructing the final kill switch plasmid, thus the characterization of which failed to be carried out smoothly. Given this problem, we intend to build a model trying to settle it.
References
- [1]Liu, H., et al., Synthetic Gene Circuits Enable Escherichia coli To Use Endogenous H(2)S as a Signaling Molecule for Quorum Sensing. ACS Synth Biol, 2019. 8(9): p. 2113-2120.
- [2]Anastassiadis, K., et al., Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E-coli, mammalian cells and mice. Disease Models & Mechanisms, 2009. 2(9-10): p. 508-515.
- [3]Simanshu, D.K., et al., Structural Basis of mRNA Recognition and Cleavage by Toxin MazF and Its Regulation by Antitoxin MazE in Bacillus subtilis. Molecular Cell, 2013. 52(3): p. 447-458.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal EcoRI site found at 1300
Illegal PstI site found at 919
Illegal PstI site found at 1607 - 12INCOMPATIBLE WITH RFC[12]Illegal EcoRI site found at 1300
Illegal NheI site found at 7
Illegal NheI site found at 30
Illegal PstI site found at 919
Illegal PstI site found at 1607 - 21INCOMPATIBLE WITH RFC[21]Illegal EcoRI site found at 1300
Illegal BglII site found at 896
Illegal BglII site found at 2482
Illegal BamHI site found at 36
Illegal BamHI site found at 543
Illegal BamHI site found at 1660
Illegal BamHI site found at 2996
Illegal XhoI site found at 2954 - 23INCOMPATIBLE WITH RFC[23]Illegal EcoRI site found at 1300
Illegal PstI site found at 919
Illegal PstI site found at 1607 - 25INCOMPATIBLE WITH RFC[25]Illegal EcoRI site found at 1300
Illegal PstI site found at 919
Illegal PstI site found at 1607 - 1000COMPATIBLE WITH RFC[1000]
| None |