Part:BBa_K3823008
Pcstr: an artificial hydrogen sulfide sensitive promoter with binding sequences of CstR
Pcstr: an artificial promoter with two kinds of binding sequences of CstR from the natural Pcstr.It is sensitive to hydrogen sulfide density.
Introduction
CstR
CstR(BBa_K3823006)is a CsoR-like sulfur transferase repressor found in Staphylococcus aureus[1], reported to react with HSnH and form sulfhydrated proteins, which can turn on their regulated genes[2] . Compared with other HSnH sensors, it shows significant response to HSnH induction.And it shows no response to H2O2, a structural analogue of HSSH and a common intracellular metabolite(Figure 1.).[3]

The structure of Pcstr
Grossoehme, N., et al. found two strong candidate tandemly repeated CstR operator sites, CstO OP1 and OP2, between the cstR and cstA genes. These sites were characterized by a run of four consecutive GC-base pairs and flanked by AT-rich regions(Figure 2.) [4].

To improve its performance in sensitivity, leaky strength, maximal strength, and strength amplitude, Liu, H., et al [1]constructed a series of combinations of PL and PR(Pcstr), and found the expression from elements 1, 4, and 5 was low and relatively stable (Figure 3), indicating that they are stringent enough for reducing leaky expression caused by background HSnH. This page is the sequence of the promoter PR3 in Figure 3.

Results
1. To figure out how CstR works by sensing H2S, we characterized the function of CstR with Pcstr using pTrchis2A-CstR-Pcstr-mKate-CpSQR(donated by Professor Liu from Shandong University). The expression level regulated by Pcstr at different concentrations of S2- was shown by the fluorescence intensity of mKate(/OD600 nm).
At the beginning, bacteria cultured in 5 mL were treated with 0, 6.5, 13.0, 26.0 and 39.0 mg/L Na2S (0, 20, 40, 80 and 120 mg/L Na2S·9H2O) respectively, and then the fluorescence intensity of mKate was measured with a microplate reader(Figure 4.).
It can be seen that the presence of S2- at different concentrations has no significant effect on the growth of bacteria. However, with the increase of S2-, the fluorescence intensity increases at first and then decreases, indicating that when S2- is too high, the lifting effect on CstR inhibition is weakened. We hope to find out an appropriate concentration range in which the strength of Pcstr is positively correlated with the concentration of S2-, which tells us that we need to reduce the concentration gradient and concentration range for further characterization.
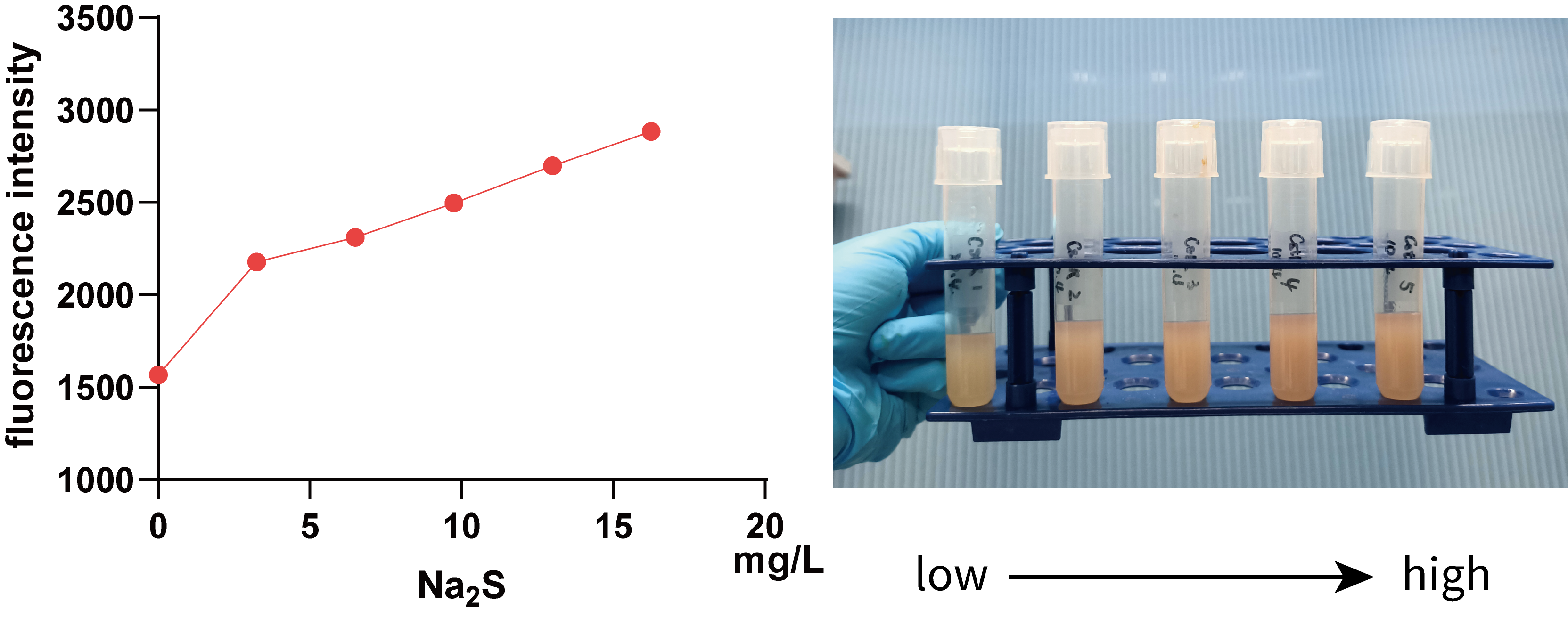
We further used 0, 3.25, 6.50, 9.75, 13.00, 16.25 mg/L Na2S (0, 10, 20, 30, 40 and 50 mg/L Na2S·9H2O) to treat 5 mL bacterial solution and then measured the fluorescence intensity of mKate using a microplate reader(Figure 5.).
It can be seen from the figure that in a smaller concentration range, there is a positive correlation between fluorescence intensity and Na2S concentration, visible to naked eyes, which implies that we can use data within this range to regulate Pcstr by giving different concentration H2S.
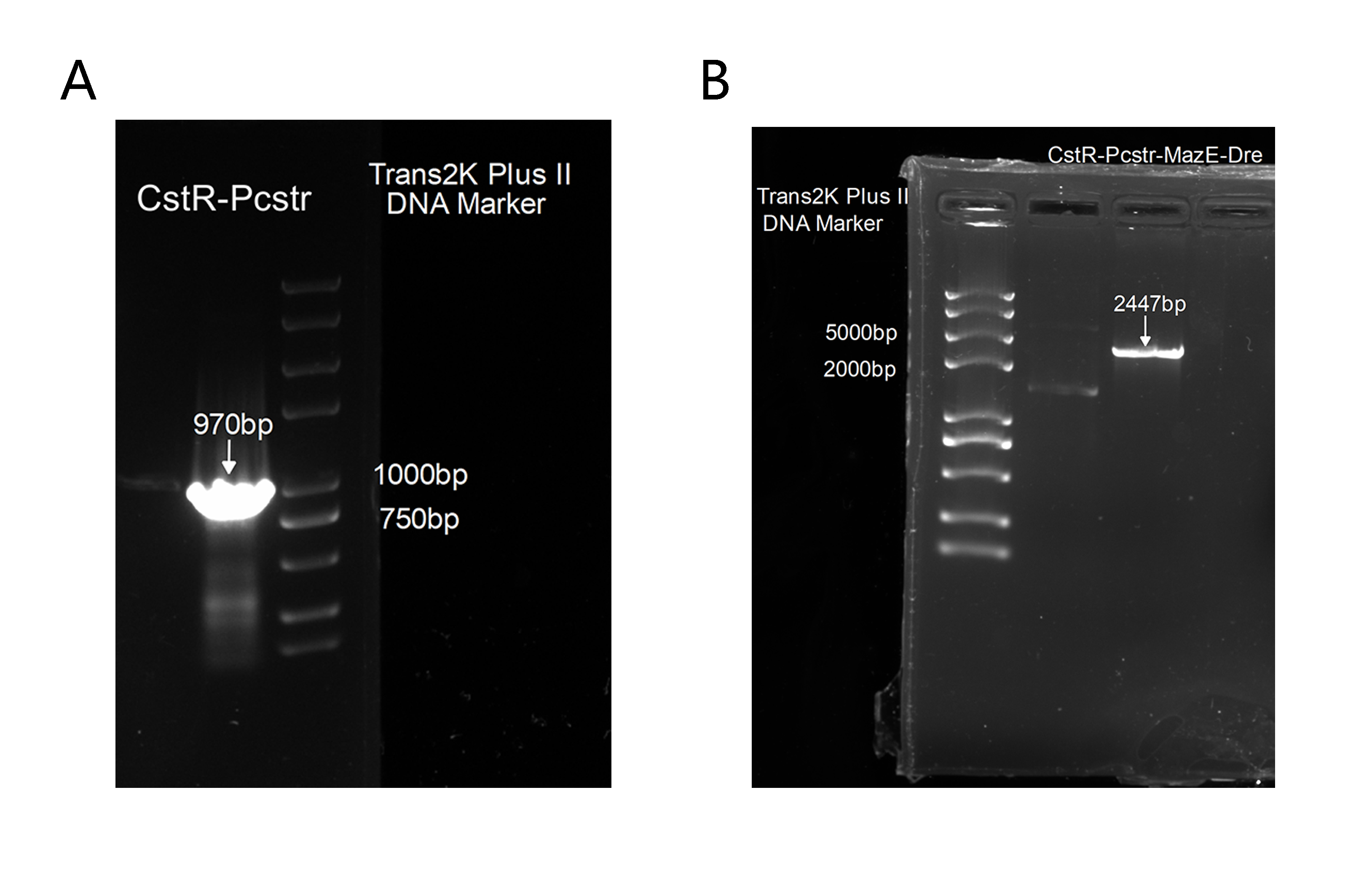
2. Also, to get this part for our plasmid construction, we successfully amplified CstR-Pcstr(Figure 6A), which was proved correct by sequencing and we has cloned it into our backbone to construct CstR-Pcstr-MazE-Dre(Figure 6B),which was also proved correct by sequencing.
However, according to the sequencing results, we found that Pcstr was missing(not in the PCR product), probably due to the complexity of each block. Meanwhile, we found the terminator between the two rox sites was partly moved after we cultured the final plasmid. We assumed that it could be the leakage of Pcstr that turned on the expression of Dre, which then cut off the terminator. This means we need to optimize our design.
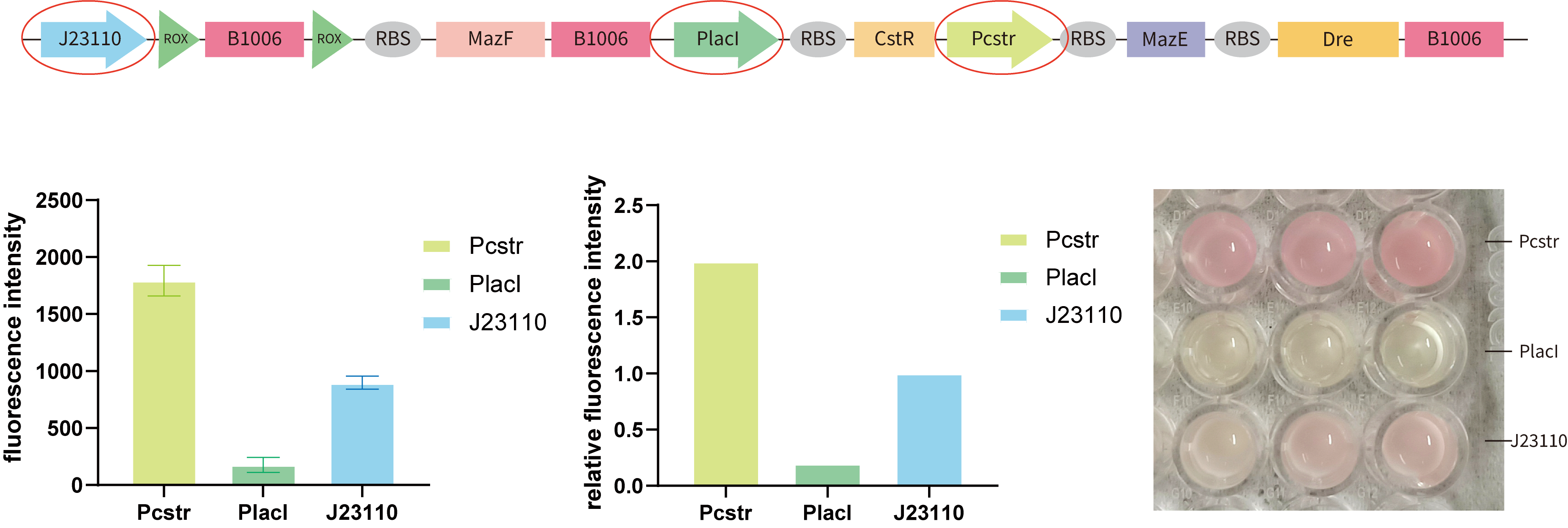
3.To optimize the design of our kill switch, we need to compare the strength of the three key promoters, PR(Pcstr), PL(PlacI), and J23110. So we tested their strength respectively by cloning them to BBa_J61002in E.coli BL21(DE3).(Figure 7.)
From our results, we found that the intensity of Pcstr is significantly higher than J23110, and PlacI is very lower than J23110. This may explain why we encountered the leakage of Pcstr —— the inhibition of the CstR may be too low to block Pcstr while the intensity of Pcstr is in a high level. Although this is not consistent with the literature, the facts tell us that it is. Considering the time limitation and the workload of changing different promoters to test the best combination, we tried to construct a model to achieve this.
References
- [1]Grossoehme, N., et al., Control of Copper Resistance and Inorganic Sulfur Metabolism by Paralogous Regulators in Staphylococcus aureus. Journal of Biological Chemistry, 2011. 286(15): p. 13522-13531.
- [2]Giedroc, D.P., A new player in bacterial sulfide-inducible transcriptional regulation. Mol Microbiol, 2017. 105(3): p. 347-352.
- [3]Liu, H., et al., Synthetic Gene Circuits Enable Escherichia coli To Use Endogenous H(2)S as a Signaling Molecule for Quorum Sensing. ACS Synth Biol, 2019. 8(9): p. 2113-2120.
- [4]Grossoehme, N., et al., Control of Copper Resistance and Inorganic Sulfur Metabolism by Paralogous Regulators in Staphylococcus aureus. Journal of Biological Chemistry, 2011. 286(15): p. 13522-13531.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal EcoRI site found at 31
- 12INCOMPATIBLE WITH RFC[12]Illegal EcoRI site found at 31
- 21INCOMPATIBLE WITH RFC[21]Illegal EcoRI site found at 31
- 23INCOMPATIBLE WITH RFC[23]Illegal EcoRI site found at 31
- 25INCOMPATIBLE WITH RFC[25]Illegal EcoRI site found at 31
- 1000COMPATIBLE WITH RFC[1000]
| None |