Part:BBa_K3781014
mVenus, MocloMania B4
This part codes for the yellow fluorescent protein with the poetic name mVenus.[1] It is a deviation of the famous green fluorescent protein GFP originally isolated from the bioluminescent jellyfish Aequorea victoria in 1962.[2] By coupling a protein of interest with a fluorescent tag such as mVenus, the target protein can be easily and non-invasively detected within living cells as well as cell preparations with the help of fluorescence spectroscopy and microscopy.[3]
size 26.9 kDa
function fluorescent tag
excitation wavelength 515 nm
emission wavelength 537 nm
cloning position B4
plasmid backbone pAGM1299
Data
We were able to successfully clone this basic part into its respective L0 plasmid backbone and to confirm the integrity of the L0 construct via restriction digest and gel electrophoresis, see Figure 1. Furthermore, we were able to include it into a L1 construct, proving its correct adaptation towards MoClo assembly, see Figure 2.
-
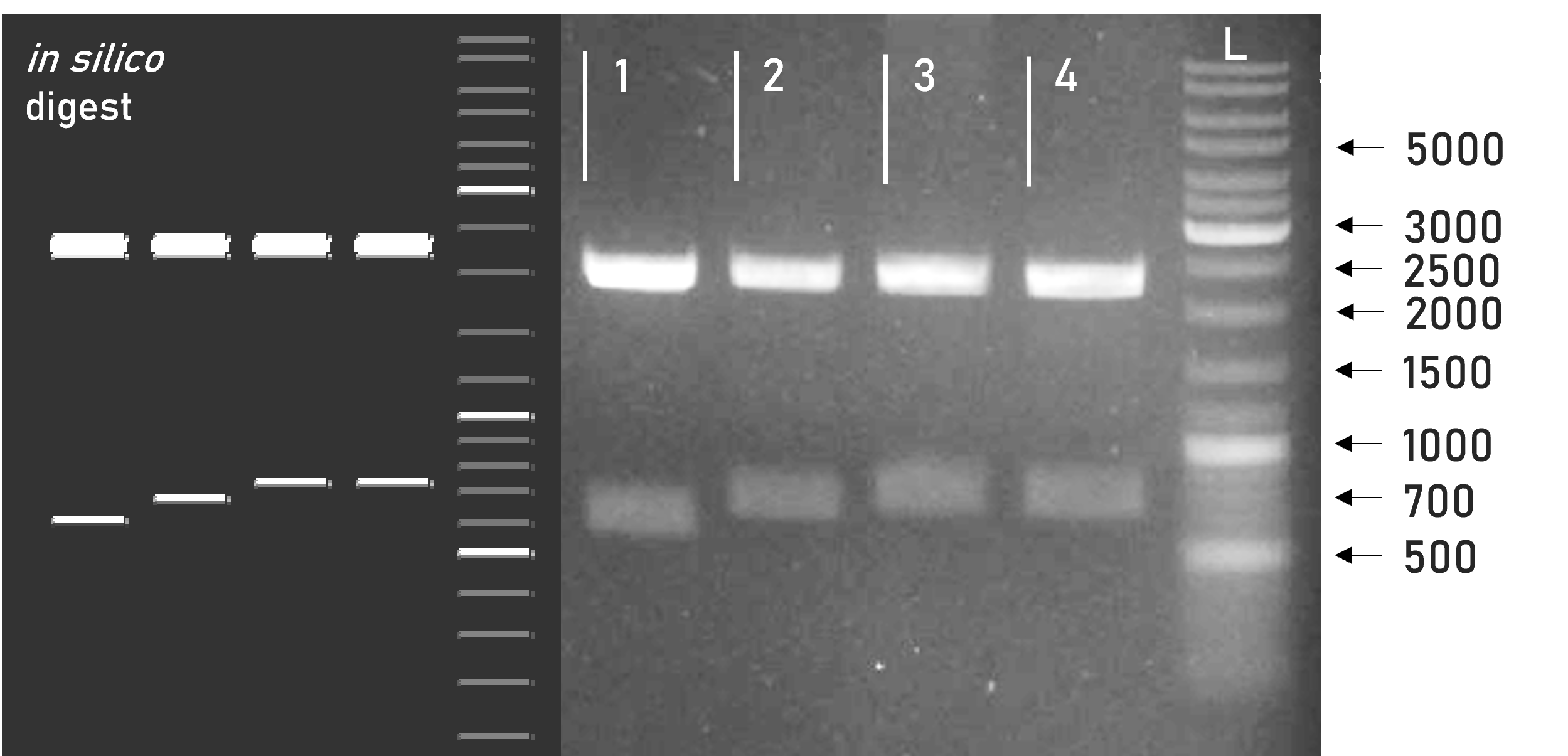 Figure 1 | Test digest of L0 B4 parts using BsaI
Figure 1 | Test digest of L0 B4 parts using BsaI
1 | pAGM1299 | 2247 + 598 bp
2 | L0_RBD_B4 | 2247 + 675 bp
3 | L0_mCerulean_B4 | 2247 + 720 bp
4 | L0_mVenus_B4 | 2247 + 720 bp
L | Thermofischer GeneRuler Plus Ladder [bp] -
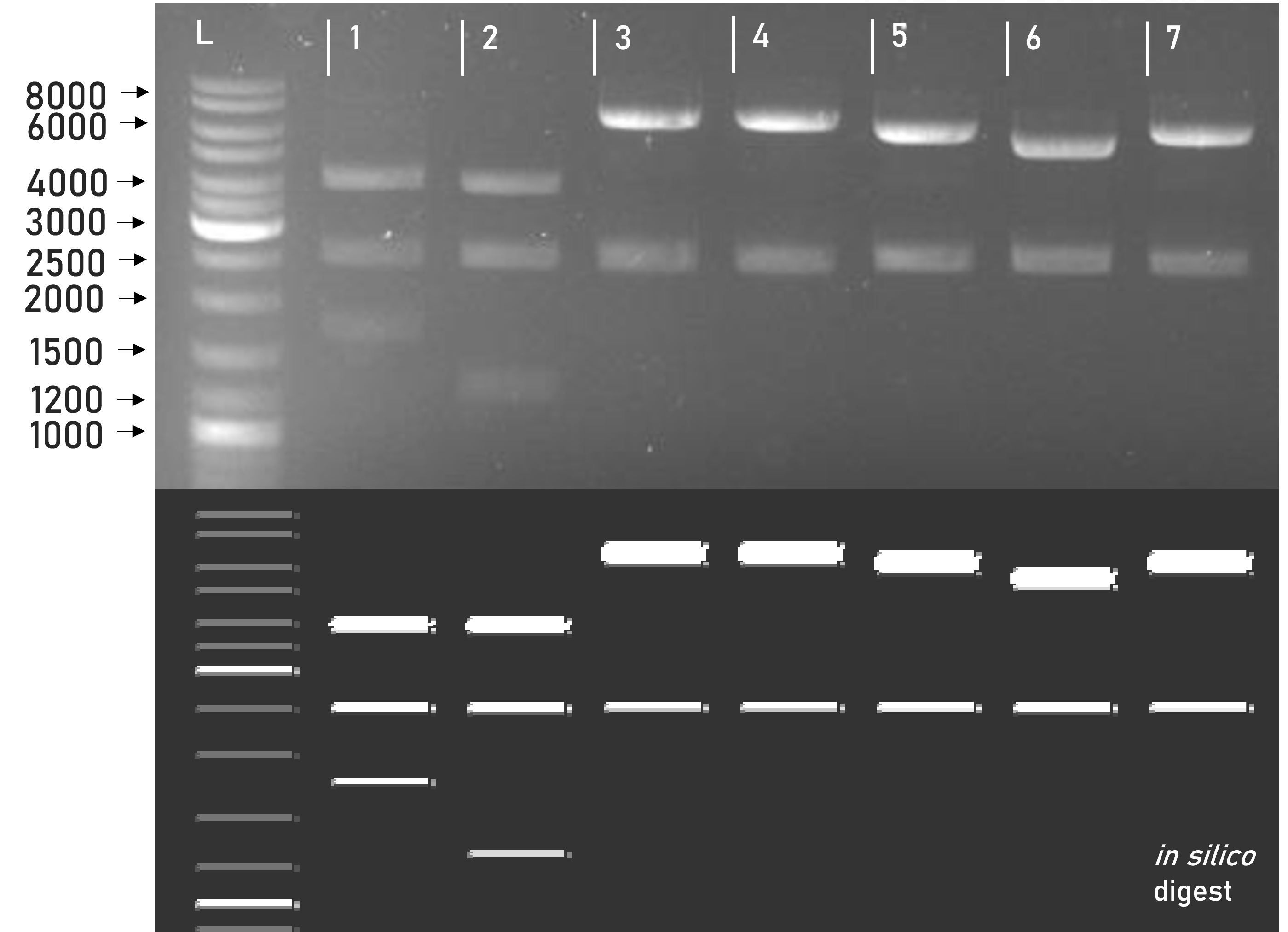 Figure 2 | Test digest of L1 constructs using SacI
Figure 2 | Test digest of L1 constructs using SacI
1 | pLEXSY_IE-blecherry3 | 3968 + 2515 + 1727 bp
2 | weird_plex | 3927 + 2515 + 1268 bp
3 | L1_sAP_RBD_mCerulean_GST | 6806 + 2515 bp
4 | L1_sAP_RBD_mVenus_GST | 6758 + 2515 bp
5 | L1_sAP_RBD_mCerulean_Strep8His | 6182 + 2515 bp
6 | L1_sAP_RBD_Strep8His | 5414 + 2515 bp
7 | L1_sAP_RBD_mVenus_Strep8His | 6134 + 2515 bp
L | Thermofischer GeneRuler Plus Ladder [bp]
Several L1 constructs assembled with L0_mVenus_B4 have been successfully transfected into Leishmania, yet resulting recombinant protein expression could only be observed on immunoblots for L1_sAP_RBD_mVenus_GST, see Figure 3.
-
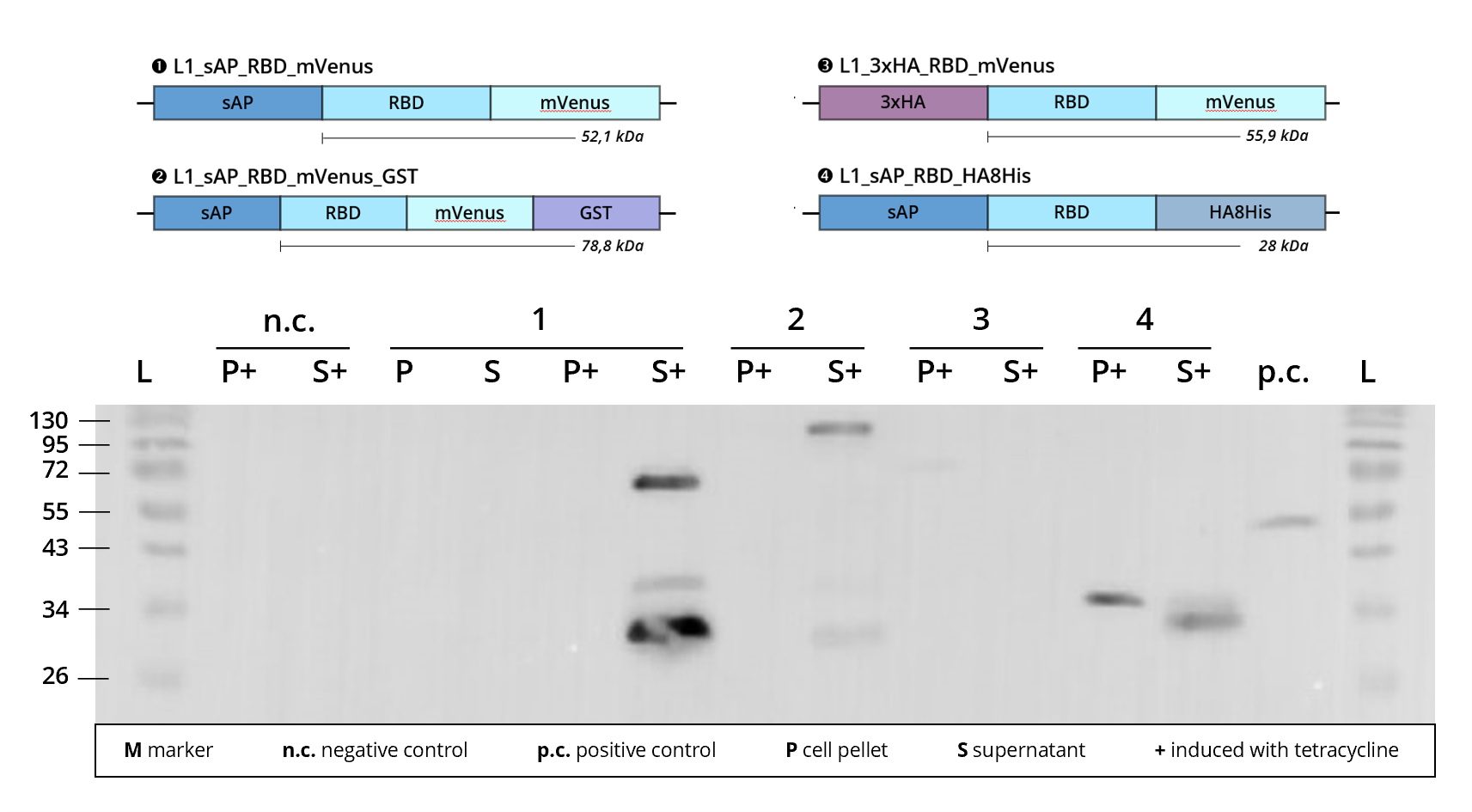 Figure 3 | Immunoblot of L1 transfected Leishmania cell cultures | stained against RBD
Figure 3 | Immunoblot of L1 transfected Leishmania cell cultures | stained against RBD
1 | L1_sAP_RBD_mVenus | 52.1 kDa
2 | L1_sAP_RBD_mVenus_GST | 78.8 kDa
3 | L1_3xHA_RBD_mVenus | 55.9 kDa
4 | L1_sAP_RBD_HA8His | 28 kDa
n.c. | negative control | Leishmania culture
transfected with empty L1 expression vector
p.c. | RBD-GFP | 52 kDa
L | Thermofischer PageRuler Protein Ladder [kDa]
1. AB | ms anti-RBD | 1:2,000
2. AB | rb anti-ms HRP | 1:10,000
Looking at Figure 3, we see that construct 2 | L1_sAP_RBD_mVenus_GST shows definite protein bands in the cell culture supernatant, S+, when stained against the SARS-CoV-2 receptor binding domain. As it contains the sAP secretion tag, protein expression is expected to be prevalent in the exterior culture medium S+. In silico calculation predicts construct 2 to weigh around 80 kDa. This is contradictory to the running hight of the bands, the band attributed to the full-length protein appearing over 100 kDa in size. Since the band pattern closely resembles the one seen in other fluorophore carrying constructs, e.g. L1_sAP_RBD_mCerulean_GST, we assume that this size shift is caused by technical deficiencies of the SDS-PAGE conduct. Shift of bands to unexplicably higher sizes might stem from a problem in gel density, caused for example by excess acrylamide concentrations.
Taking into consideration the assumed deficiencies in gel density, the construct's upper band can be attributed to the full-length fusion protein. However, a smaller band, running at 30 kDa (assumed to be at ca. 27 kDa) can be observed. Since this smaller band is also detected with anti-RBD antibodies, it is hypothesized that extracellular cleavage processes separate the RBD from its fusion tags after secretion of the fusion protein into the culture medium. For more information on this, please consult the sAP secretion tag part site.
Out of all the L1 constructs employing L0_mVenus_B4, so far L1_sAP_RBD_mVenus_GST has been successfully purified. This purification didn't yield sufficient amounts of intact fusion protein for downstream analysis, because we are struggling with premature cleavage of our GST fusion tag. Therefore, fluorescence spectroscopy of purified protein samples has not yet been conducted, neither has fluorescence microscopy of Leishmania producing recombinant mVenus. Thus, at this point in time, functionality of L0_mVenus_B4 protein cannot be definitively verified. We are working hard on optimizing expression and purification yields in order to finalize part validation and implementation towards their respective functionality.
The MocloMania collection
This basic part is part of the MocloMania collection, the very first collection of genetic parts specifically designed and optimized for Modular Cloning assembly and recombinant protein expression in the protozoan parasite Leishmania tarentolae.
Are you trying to express complexly glycosylated proteins? Large antibody side chains? Human proteins that require accurate post-translational modification? Then Leishmania might be just the right organism for you! Leishmania tarentolae’s glycosylation patterns resemble those of human cells more closely than any other microbial expression host, while still delivering all the benefits of microbial production systems like easy transfection and cultivation.[4] So instead of relying on mammalian cell lines, try considering Leishmania as your new expression host of choice!
Our MocloMania collection will allow you to easily modify your protein of choice and make it suitable for downstream detection and purification procedures - all thanks to the help of Modular Cloning. This cloning system was first established by Weber et al. in 2011 and relies on the ability of type IIS restriction enzymes to cut DNA outside of their recognition sequence, hereby generating four nucleotide overhangs.[5] Every basic part in our collection is equipped with a specified set of overhangs that assign it to its designated position within the reading frame. These so-called cloning positions are labelled B2-B5 from upstream to downstream. By filling all positions with the basic parts of your choice, you can easily generate variable genetic constructs that code for the fusion protein of your desire.
We furthermore provide a specifically domesticated Leishmania expression vector, named weird_plex, which will package your fusion construct into a functional transcriptional unit that is optimized for high expression in Leishmania.
The best part? Because of the type IIS restriction properties and the specifity of the generated overhangs, restriction and ligation of your construct can all happen simultaneously in a simple one-step, one-pot reaction. This will safe you a lot of time and frustration in your cloning endeavours!
Do we have your attention? In the table below you can find some basic information on how our cloning system, along with most other MoClo systems, is set up. Please feel free to check out our wiki to find more information on Leishmania and Modular Cloning as well as to understand how this basic part integrates into our part collection. See you there!
| Level | What does this level contain? | antibiotic resistance | Enzyme used for ligation |
| L0 | The foundation to every MoClo construct which are basic genetic units, such as coding sequences, promoters, terminators | spectinomycin | BbsI |
| L1 | Several L0 parts assembled into a functional transcriptional unit, e.g. consisting of promoter, coding region and terminator | ampicillin | BsaI |
| L2 | Multiple transcriptional units added into one multi-gene construct, e.g. a protein of interest fused to a resistance cassette | kanamycin | BbsI |
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 202
- 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 202
- 21COMPATIBLE WITH RFC[21]
- 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 202
- 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 202
Illegal NgoMIV site found at 679 - 1000COMPATIBLE WITH RFC[1000]
Reference Literature
- ↑ Gert-Jan Kremers, Joachim Goedhart, Erik B. van Munster, and Theodorus W. J. Gadella. Cyan and Yellow Super Fluorescent Proteins with Improved Brightness, Protein Folding, and FRET Förster Radius, Biochemistry 2006 45 (21), 6570-6580, DOI: 10.1021/bi0516273
- ↑ Shimomura, O., Johnson, F.H. and Saiga, Y. (1962) Extraction, Purification, and Properties of Aequorin, a Bioluminescent Protein from the Luminous Hydromedusan, Aequorea. Journal of Cellular and Comparative Physiology, 59, 223-239. http://dx.doi.org/10.1002/jcp.1030590302
- ↑ M.A. Rizzo, D.W. Piston High-contrast imaging of fluorescent protein FRET by fluorescence polarization microscopy Biophys. J., 88 (2005), pp. L14-L16
- ↑ Langer T, Corvey C, Kroll K, Boscheinen O, Wendrich T, Dittrich W. Expression and purification of the extracellular domains of human glycoprotein VI (GPVI) and the receptor for advanced glycation end products (RAGE) from Rattus norvegicus in Leishmania tarentolae. Prep Biochem Biotechnol. 2017 Nov 26;47(10):1008-1015. doi: 10.1080/10826068.2017.1365252. Epub 2017 Aug 31. PMID: 28857681.
- ↑ Weber E, Engler C, Gruetzner R, Werner S, Marillonnet S (2011) A Modular Cloning System for Standardized Assembly of Multigene Constructs. PLoS ONE 6(2): e16765. https://doi.org/10.1371/journal.pone.0016765
| None |