Part:BBa_K3254014
Optimized Ptac promoter
This part is a twin of BBa_K2572025, we built this page just for more detailed sequence annotations. This promoter was optimized from Ptac promoter(BBa_K864400) through using a symmetrical lacO site. After combined with the lacI constitutive expression cassette(BBa_K3254022), it can be regulated by the IPTG inducer. A mutant library with different dynamic range had been built(see BBa_K3254015,BBa_K3254016 and BBa_K3254017).
Thermodynamic Characterization
- You can find the same contents below on the page of BBa_K2572025.
- This part can be seen as an improved version of the original Ptac promoter (BBa_K864400). The main improvement point was this version could be applied a symmetrical lacO site to get a more tightly regulation.
- The page of a twin part was build for a full annotation (BBa_K3254014).
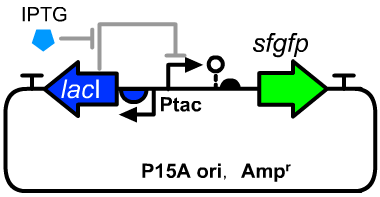
- We used this part to construct an IPTG inducible transcriptional device by combining with a lacI expression cassette(BBa_K3254022) on a P15A plasmid. An insulated sfgfp reporter translational unit (BBa_K3254024) was applied for quantitative assay the dynamic response curve. The full structure of this operon was described on the page of BBa_K3254025.
- Furthermore, we improved this part by mutant the -10 region of this promoter and successfully achieved a series of IPTG inducible promoters(Ptac-M1, Ptac-M2, Ptac-M3) with different dynamic ranges and lower non-induced activities.
Genetic Design
- The sequence detail was described on the page of BBa_K3254025.
- The host cell was E.coli DH5α.
Experimental Setup
- All incubations were carried out using a Digital Thermostatic Shaker maintained at 37 °C and 1000 rpm, using Corning flat-bottom 96-well plates sealed with sealing film. For characterizing the circuit response functions, a previously developed quantitative method that measures gene expression at steady state was used(Zhang, Chen et al. 2016). Briefly, bacteria harboring the parts/circuits of interest were first inoculated from single colonies into a flat-bottom 96-well plate for overnight growth, after which the cell cultures were diluted 196-fold with M9 medium. After 3 h of growth, the cultures were further diluted 700-fold with M9 medium containing gradient concentrations of IPTG, and incubated for another 6 h. Finally, 20-μL samples of each culture were transferred to a new plate containing 180 μL per well of PBS supplemented with 2 mg/mL kanamycin to terminate protein expression. The fluorescence distribution of each sample was assayed using a flow cytometer with appropriate voltage settings; each distribution contained >20,000 events. Each sample was experimentally assayed at least three times. The arithmetical mean of each sample was determined using FlowJo software.
- M9 medium (supplemented): 6.8 g/L Na2HPO4, 3 g/L KH2PO4, 0.5 g/L NaCl, 1 g/L NH4Cl, 0.34 g/L thiamine, 0.2% casamino acids, 0.4% glucose, 2mM MgSO4, and 100 μM CaCl2.
Results
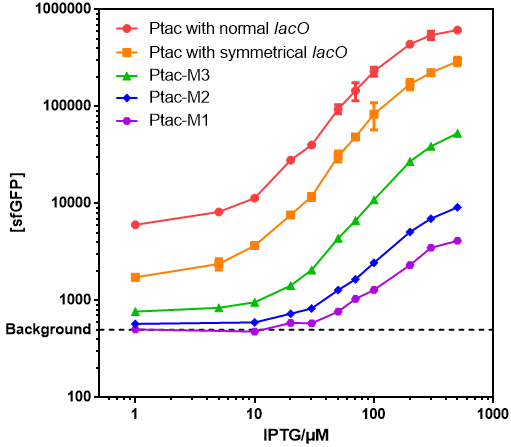
- Those promoters have gentle response curves without saltus.
- Background: Cells without any FP genes.
- Ptac with normal lacO: BBa_K864400
- Ptac with symmetrical lacO: BBa_K2572025
- Ptac-M1: BBa_K3254015
- Ptac-M2: BBa_K3254016
- Ptac-M3: BBa_K3254017
References
- Zhang HM, et al. Measurements of Gene Expression at Steady State Improve the Predictability of Part Assembly. ACS Synthetic Biology 5, 269-273 (2016).
- Zong Y, et al. Insulated transcriptional elements enable precise design of genetic circuits. Nature communications 8, 52 (2017).
Contribution: HKUST 2022
Team HKUST 2022 uses pTac for their experiment on the characterization of their degradation system using TEV. For more information on how HKUST 2022 Team uses pTac, refer to the composite part BBa_K4225012 and BBa_K4225019
Sequence and Features BBa_K3254014 Sequence And Features Not understood
//direction/forward
//promoter
//regulation/positive
//rnap/prokaryote/ecoli/sigma70
| None |