Part:BBa_K2017003
SAGTI linker + Luciferase
Firefly luciferase protein. It oxidates its substrate luciferin, emitting light during the process. It can act as a reporter protein when used with a promoter. This part is made to be fused downstream to other coding sequence, so it includes the SAGTI linker in order to let the luciferase acquire the correct tertiary structure.
The luciferase does not include ATG (Met) to initiate translation, as it needs to be fused to other coding sequence on the 5'. The translation must begin in the coding sequence fused upstream. The linker includes a random nucleotide in 5', to change the reading frame of the luciferase. That way, it will not be translated unless an indel is produced in the coding region to which the part is fused.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 822
Usage and Biology
Luciferase protein is a useful reporter. It emits light when it oxidates luciferin. In our gRNA testing system, we need to have a truncated luciferase. We have inserted an additional nucleotide before the coding sequence, and we have removed the ATG (Met) that initiates translation. This avoids the luciferase to be expressed unless it is put back to its reading frame. This occurs when it is inserted in our [http://2016.igem.org/Team:Valencia_UPV/Hardware/Reagents#Ourdevice_id gRNA testing system device] and the CRISPR/Cas9 cuts the target. Upstream the luciferase, it is put a linker. In the gRNA testing system, when the device is transcribed and translated, the gene target is attached to the luciferase. When the protein folds, the gene target could interact with the luciferase and affect its tertiary structure. The upstream linker avoids this by creating a distance between the gene target and the luciferase. The linker, theoretically, does not interact with the luciferase.
This part is one of the standard parts that can be used to create our gRNA testing system device, together with a promoter, terminator and a gene target of your choice.
Characterization
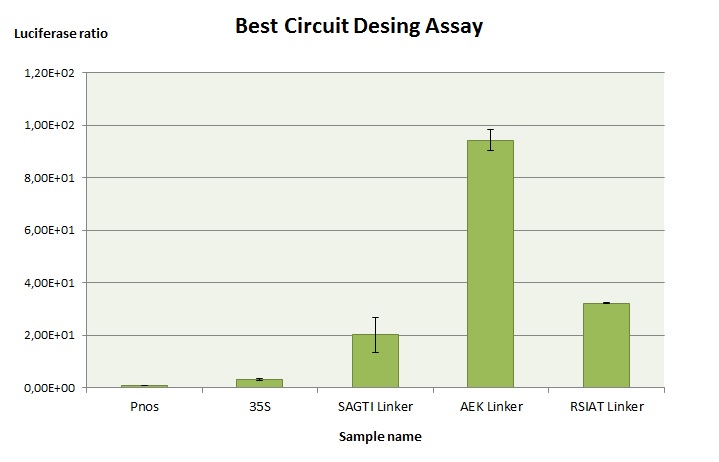
To optimize the gRNA testing system, we tried with three different linkers (BBa_K201703, BBa_K201704 and BBa_K201705. Our objective was to simulate how well the luciferase would work in the case that the Cas9 would cut the gene target. As the most common editing of the Cas9 according to bibliography was one deletion, for this experiment we designed the target as if it has been cut by the Cas9. This way, the luciferase should be expressed.
The experiment consists on agroinfiltrating the gRNA testing system with a non-useful gRNA (it does not cut the target because we designed it as if it has been cut) and 35s:Renilla:Pnos, to normalize the data. Afterwards, a luciferase essay is performed according to our [http://2016.igem.org/Team:Valencia_UPV/Notebook/Protocol#Luciferaseassay_id protocol].
35s (35s:Luciferase:Tnos) is used as positive control in luciferase essays. Pnos (pNOS:Luciferase:Tnos) is used as basal control. As it can seen, the linker that allows luciferase to have higher activity is AEK linker BBa_K201705.
This demonstrates that our gRNA testing system device can be functional when the Cas9 cuts the target gene and puts it back to the correct reading frame.
References
Holden M, Levine M, Scholdberg T, Haynes R, Jenkins G. The use of 35S and Tnos expression elements in the measurement of genetically engineered plant materials. Analytical and Bioanalytical Chemistry. 2009;396(6):2175-2187.
//chassis/eukaryote/plants/other
| None |